Immunity Against Fungi
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Estimated Burden of Serious Fungal Infections in Ghana
Journal of Fungi Article Estimated Burden of Serious Fungal Infections in Ghana Bright K. Ocansey 1, George A. Pesewu 2,*, Francis S. Codjoe 2, Samuel Osei-Djarbeng 3, Patrick K. Feglo 4 and David W. Denning 5 1 Laboratory Unit, New Hope Specialist Hospital, Aflao 00233, Ghana; [email protected] 2 Department of Medical Laboratory Sciences, School of Biomedical and Allied Health Sciences, College of Health Sciences, University of Ghana, P.O. Box KB-143, Korle-Bu, Accra 00233, Ghana; [email protected] 3 Department of Pharmaceutical Sciences, Faculty of Health Sciences, Kumasi Technical University, P.O. Box 854, Kumasi 00233, Ghana; [email protected] 4 Department of Clinical Microbiology, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi 00233, Ghana; [email protected] 5 National Aspergillosis Centre, Wythenshawe Hospital and the University of Manchester, Manchester M23 9LT, UK; [email protected] * Correspondence: [email protected] or [email protected] or [email protected]; Tel.: +233-277-301-300; Fax: +233-240-190-737 Received: 5 March 2019; Accepted: 14 April 2019; Published: 11 May 2019 Abstract: Fungal infections are increasingly becoming common and yet often neglected in developing countries. Information on the burden of these infections is important for improved patient outcomes. The burden of serious fungal infections in Ghana is unknown. We aimed to estimate this burden. Using local, regional, or global data and estimates of population and at-risk groups, deterministic modelling was employed to estimate national incidence or prevalence. Our study revealed that about 4% of Ghanaians suffer from serious fungal infections yearly, with over 35,000 affected by life-threatening invasive fungal infections. -

Introduction
Notes Introduction 1. In the book, we use the terms ‘fungal infections’ and ‘mycoses’ (or singular fungal infection and mycosis) interchangeably, mostly following the usage of our historical actors in time and place. 2. The term ‘ringworm’ is very old and came from the circular patches of peeled, inflamed skin that characterised the infection. In medicine at least, no one understood it to be associated with worms of any description. 3. Porter, R., ‘The patient’s view: Doing medical history from below’, Theory and Society, 1985, 14: 175–198; Condrau, F., ‘The patient’s view meets the clinical gaze’, Social History of Medicine, 2007, 20: 525–540. 4. Burnham, J. C., ‘American medicine’s golden age: What happened to it?’, Sci- ence, 1982, 215: 1474–1479; Brandt, A. M. and Gardner, M., ‘The golden age of medicine’, in Cooter, R. and Pickstone, J. V., eds, Medicine in the Twentieth Century, Amsterdam, Harwood, 2000, 21–38. 5. The Oxford English Dictionary states that the first use of ‘side-effect’ was in 1939, in H. N. G. Wright and M. L. Montag’s textbook: Materia Med Pharma- col & Therapeutics, 112, when it referred to ‘The effects which are not desired in any particular case are referred to as “side effects” or “side actions” and, in some instances, these may be so powerful as to limit seriously the therapeutic usefulness of the drug.’ 6. Illich, I., Medical Nemesis: The Expropriation of Health, London, Calder & Boyars, 1975. 7. Beck, U., Risk Society: Towards a New Society, New Delhi, Sage, 1992, 56 and 63; Greene, J., Prescribing by Numbers: Drugs and the Definition of Dis- ease, Baltimore, Johns Hopkins University Press, 2007; Timmermann, C., ‘To treat or not to treat: Drug research and the changing nature of essential hypertension’, Schlich, T. -

Microglial Function and Regulation During Development, Homeostasis and Alzheimer’S Disease
cells Review Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease Brad T. Casali and Erin G. Reed-Geaghan * Department of Pharmaceutical Sciences, Northeast Ohio Medical University, Rootstown, OH 44272, USA; [email protected] * Correspondence: [email protected] Abstract: Microglia are the resident immune cells of the brain, deriving from yolk sac progenitors that populate the brain parenchyma during development. During development and homeostasis, microglia play critical roles in synaptogenesis and synaptic plasticity, in addition to their primary role as immune sentinels. In aging and neurodegenerative diseases generally, and Alzheimer’s disease (AD) specifically, microglial function is altered in ways that significantly diverge from their homeostatic state, inducing a more detrimental inflammatory environment. In this review, we discuss the receptors, signaling, regulation and gene expression patterns of microglia that mediate their phenotype and function contributing to the inflammatory milieu of the AD brain, as well as strategies that target microglia to ameliorate the onset, progression and symptoms of AD. Keywords: microglia; inflammation; Alzheimer’s disease; neurodegenerative diseases; TREM2; neu- roinflammation Citation: Casali, B.T.; Reed-Geaghan, 1. Introduction E.G. Microglial Function and Microglia are the resident phagocytes of the central nervous system (CNS). In addition Regulation during Development, to their immunological role in maintaining CNS homeostasis, microglia play vital -

Standard Methods for Fungal Brood Disease Research Métodos Estándar Para La Investigación De Enfermedades Fúngicas De La Cr
Journal of Apicultural Research 52(1): (2013) © IBRA 2013 DOI 10.3896/IBRA.1.52.1.13 REVIEW ARTICLE Standard methods for fungal brood disease research Annette Bruun Jensen1*, Kathrine Aronstein2, José Manuel Flores3, Svjetlana Vojvodic4, María 5 6 Alejandra Palacio and Marla Spivak 1Department of Plant and Environmental Sciences, University of Copenhagen, Thorvaldsensvej 40, 1817 Frederiksberg C, Denmark. 2Honey Bee Research Unit, USDA-ARS, 2413 E. Hwy. 83, Weslaco, TX 78596, USA. 3Department of Zoology, University of Córdoba, Campus Universitario de Rabanales (Ed. C-1), 14071, Córdoba, Spain. 4Center for Insect Science, University of Arizona, 1041 E. Lowell Street, PO Box 210106, Tucson, AZ 85721-0106, USA. 5Unidad Integrada INTA – Facultad de Ciencias Ags, Universidad Nacional de Mar del Plata, CC 276,7600 Balcarce, Argentina. 6Department of Entomology, University of Minnesota, St. Paul, Minnesota 55108, USA. Received 1 May 2012, accepted subject to revision 17 July 2012, accepted for publication 12 September 2012. *Corresponding author: Email: [email protected] Summary Chalkbrood and stonebrood are two fungal diseases associated with honey bee brood. Chalkbrood, caused by Ascosphaera apis, is a common and widespread disease that can result in severe reduction of emerging worker bees and thus overall colony productivity. Stonebrood is caused by Aspergillus spp. that are rarely observed, so the impact on colony health is not very well understood. A major concern with the presence of Aspergillus in honey bees is the production of airborne conidia, which can lead to allergic bronchopulmonary aspergillosis, pulmonary aspergilloma, or even invasive aspergillosis in lung tissues upon inhalation by humans. In the current chapter we describe the honey bee disease symptoms of these fungal pathogens. -
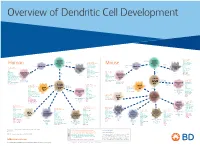
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit) -

Human Lectins, Their Carbohydrate Affinities and Where to Find Them
biomolecules Review Human Lectins, Their Carbohydrate Affinities and Where to Review HumanFind Them Lectins, Their Carbohydrate Affinities and Where to FindCláudia ThemD. Raposo 1,*, André B. Canelas 2 and M. Teresa Barros 1 1, 2 1 Cláudia D. Raposo * , Andr1 é LAQVB. Canelas‐Requimte,and Department M. Teresa of Chemistry, Barros NOVA School of Science and Technology, Universidade NOVA de Lisboa, 2829‐516 Caparica, Portugal; [email protected] 12 GlanbiaLAQV-Requimte,‐AgriChemWhey, Department Lisheen of Chemistry, Mine, Killoran, NOVA Moyne, School E41 of ScienceR622 Co. and Tipperary, Technology, Ireland; canelas‐ [email protected] NOVA de Lisboa, 2829-516 Caparica, Portugal; [email protected] 2* Correspondence:Glanbia-AgriChemWhey, [email protected]; Lisheen Mine, Tel.: Killoran, +351‐212948550 Moyne, E41 R622 Tipperary, Ireland; [email protected] * Correspondence: [email protected]; Tel.: +351-212948550 Abstract: Lectins are a class of proteins responsible for several biological roles such as cell‐cell in‐ Abstract:teractions,Lectins signaling are pathways, a class of and proteins several responsible innate immune for several responses biological against roles pathogens. such as Since cell-cell lec‐ interactions,tins are able signalingto bind to pathways, carbohydrates, and several they can innate be a immuneviable target responses for targeted against drug pathogens. delivery Since sys‐ lectinstems. In are fact, able several to bind lectins to carbohydrates, were approved they by canFood be and a viable Drug targetAdministration for targeted for drugthat purpose. delivery systems.Information In fact, about several specific lectins carbohydrate were approved recognition by Food by andlectin Drug receptors Administration was gathered for that herein, purpose. plus Informationthe specific organs about specific where those carbohydrate lectins can recognition be found by within lectin the receptors human was body. -

Tinea Capitis 2014 L.C
BJD GUIDELINES British Journal of Dermatology British Association of Dermatologists’ guidelines for the management of tinea capitis 2014 L.C. Fuller,1 R.C. Barton,2 M.F. Mohd Mustapa,3 L.E. Proudfoot,4 S.P. Punjabi5 and E.M. Higgins6 1Department of Dermatology, Chelsea & Westminster Hospital, Fulham Road, London SW10 9NH, U.K. 2Department of Microbiology, Leeds General Infirmary, Leeds LS1 3EX, U.K. 3British Association of Dermatologists, Willan House, 4 Fitzroy Square, London W1T 5HQ, U.K. 4St John’s Institute of Dermatology, Guy’s and St Thomas’ NHS Foundation Trust, St Thomas’ Hospital, Westminster Bridge Road, London SE1 7EH, U.K. 5Department of Dermatology, Hammersmith Hospital, 150 Du Cane Road, London W12 0HS, U.K. 6Department of Dermatology, King’s College Hospital, Denmark Hill, London SE5 9RS, U.K. 1.0 Purpose and scope Correspondence Claire Fuller. The overall objective of this guideline is to provide up-to- E-mail: [email protected] date, evidence-based recommendations for the management of tinea capitis. This document aims to update and expand Accepted for publication on the previous guidelines by (i) offering an appraisal of 8 June 2014 all relevant literature since January 1999, focusing on any key developments; (ii) addressing important, practical clini- Funding sources cal questions relating to the primary guideline objective, i.e. None. accurate diagnosis and identification of cases; suitable treat- ment to minimize duration of disease, discomfort and scar- Conflicts of interest ring; and limiting spread among other members of the L.C.F. has received sponsorship to attend conferences from Almirall, Janssen and LEO Pharma (nonspecific); has acted as a consultant for Alliance Pharma (nonspe- community; (iii) providing guideline recommendations and, cific); and has legal representation for L’Oreal U.K. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -

Therapies for Common Cutaneous Fungal Infections
MedicineToday 2014; 15(6): 35-47 PEER REVIEWED FEATURE 2 CPD POINTS Therapies for common cutaneous fungal infections KENG-EE THAI MB BS(Hons), BMedSci(Hons), FACD Key points A practical approach to the diagnosis and treatment of common fungal • Fungal infection should infections of the skin and hair is provided. Topical antifungal therapies always be in the differential are effective and usually used as first-line therapy, with oral antifungals diagnosis of any scaly rash. being saved for recalcitrant infections. Treatment should be for several • Topical antifungal agents are typically adequate treatment weeks at least. for simple tinea. • Oral antifungal therapy may inea and yeast infections are among the dermatophytoses (tinea) and yeast infections be required for extensive most common diagnoses found in general and their differential diagnoses and treatments disease, fungal folliculitis and practice and dermatology. Although are then discussed (Table). tinea involving the face, hair- antifungal therapies are effective in these bearing areas, palms and T infections, an accurate diagnosis is required to ANTIFUNGAL THERAPIES soles. avoid misuse of these or other topical agents. Topical antifungal preparations are the most • Tinea should be suspected if Furthermore, subsequent active prevention is commonly prescribed agents for dermatomy- there is unilateral hand just as important as the initial treatment of the coses, with systemic agents being used for dermatitis and rash on both fungal infection. complex, widespread tinea or when topical agents feet – ‘one hand and two feet’ This article provides a practical approach fail for tinea or yeast infections. The pharmacol- involvement. to antifungal therapy for common fungal infec- ogy of the systemic agents is discussed first here. -

Immunology Focus Summer | 2006 Immunology FOCUS
R&D Systems Immunology Focus summer | 2006 Immunology FOCUS Inside page 2 Signal Transduction: Kinase & Phosphatase Reagents page 3 Lectin Family page 4 Regulatory T Cells page 5 Natural Killer Cells page 6 Innate Immunity & Dendritic Cells page 7 Co-Stimulation/-Inhibition The B7 Family & Associated Molecules page 8 Proteome Profiler™ Phospho-Immunoreceptor Array ITAM/ITIM-Associated Receptors www.RnDSystems.com Please visit our website @ www.RnDSystems.com for product information and past issues of the Focus Newsletter: Cancer, Neuroscience, Cell Biology, and more. Quality | Selec tion | Pe rformance | Result s Cancer Development Endocrinology Immunology Neuroscience Proteases Stem Cells Signal Transduction: Kinase & Phosphatase Reagents Co-inhibitory PD-L2/PD-1 signaling & SHP-2 phosphatase KINASE & PHOSPHATASE RESEARCH REAGENTS Regulation of MAP kinase (MAPK) signaling Kinases Phosphatases pathways is critical for T cell development, MOLECULE ANTIBODIES ELISAs/ASSAYS MOLECULE ANTIBODIES ELISAs/ASSAYS activation, differentiation, and death. MAPKs Akt Family H M R H M R Alkaline Phosphatase* H M R are activated by the dual phosphorylation of threonine and tyrosine residues resulting in AMPK H M R Calcineurin A, B H M R subsequent transcription factor activation. ATM H M R H CD45 H M H M The MAPK signaling pathway in T cells can be CaM Kinase II Ms CDC25A, B* H M R triggered by cytokines, growth factors, and CDC2 H M R DARPP-32 M R ligands for transmembrane receptors. Chk1, 2 H M R H M R DEP-1/CD148* H M R H Ligation of the T cell receptor (TCR)/CD3 ERK1, 2 H M R H M R LAR H M R complex results in rapid activation of PI 3- kinase, which leads to Akt and MAPK ERK3 H Lyp H activation. -

Type I Interferon Response Drives Neuroinflammation and Synapse Loss in Alzheimer Disease
RESEARCH ARTICLE The Journal of Clinical Investigation Type I interferon response drives neuroinflammation and synapse loss in Alzheimer disease Ethan R. Roy,1,2 Baiping Wang,1 Ying-wooi Wan,3 Gabriel Chiu,1 Allysa Cole,1 Zhuoran Yin,4 Nicholas E. Propson,1,5 Yin Xu,1 Joanna L. Jankowsky,6 Zhandong Liu,7 Virginia M.-Y. Lee,8 John Q. Trojanowski,8 Stephen D. Ginsberg,9,10 Oleg Butovsky,4,11 Hui Zheng,1,3 and Wei Cao1,3 1Huffington Center on Aging, 2Translational Biology & Molecular Medicine Program, and 3Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, Texas, USA. 4Ann Romney Center for Neurologic Diseases, Department of Neurology, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. 5Molecular and Cellular Biology Program, Department of Molecular and Cellular Biology, 6Department of Neuroscience, and 7Department of Pediatrics-Neurology, Baylor College of Medicine, Houston, Texas, USA. 8Department of Pathology and Laboratory Medicine, University of Pennsylvania School of Medicine, Philadelphia, Pennsylvania, USA. 9Center for Dementia Research, Nathan Kline Institute, Orangeburg, New York, USA. 10Departments of Psychiatry, Neuroscience & Physiology and the NYU Neuroscience Institute, New York University Langone Medical Center, New York, New York, USA. 11Evergrande Center for Immunologic Diseases, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts, USA. Type I interferon (IFN) is a key cytokine that curbs viral infection and cell malignancy. Previously, we demonstrated a potent IFN immunogenicity of nucleic acid–containing (NA-containing) amyloid fibrils in the periphery. Here, we investigated whether IFN is associated with β-amyloidosis inside the brain and contributes to neuropathology. -

Influence of Galectin-3 on the Innate Immune Response During
Journal of Fungi Article Influence of Galectin-3 on the Innate Immune Response during Experimental Cryptococcosis Caroline Patini Rezende 1, Patricia Kellen Martins Oliveira Brito 2 , Thiago Aparecido Da Silva 2 , Andre Moreira Pessoni 1 , Leandra Naira Zambelli Ramalho 3 and Fausto Almeida 1,* 1 Department of Biochemistry and Immunology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] (C.P.R.); [email protected] (A.M.P.) 2 Department of Cellular and Molecular Biology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] (P.K.M.O.B.); [email protected] (T.A.D.S.) 3 Department of Pathology, Ribeirao Preto Medical School, University of Sao Paulo, Ribeirao Preto 14049-900, SP, Brazil; [email protected] * Correspondence: [email protected] Abstract: Cryptococcus neoformans, the causative agent of cryptococcosis, is the primary fungal pathogen that affects the immunocompromised individuals. Galectin-3 (Gal-3) is an animal lectin involved in both innate and adaptive immune responses. The present study aimed to evaluate the influence of Gal-3 on the C. neoformans infection. We performed histopathological and gene profile analysis of the innate antifungal immunity markers in the lungs, spleen, and brain of the wild-type (WT) and Gal-3 knockout (KO) mice during cryptococcosis. These findings suggest that Gal-3 absence does not cause significant histopathological alterations in the analyzed tissues. The expression profile of the genes related to innate antifungal immunity showed that the presence of cryptococcosis in Citation: Rezende, C.P.; Brito, the WT and Gal-3 KO animals, compared to their respective controls, promoted the upregulation P.K.M.O.; Da Silva, T.A.; Pessoni, of the pattern recognition receptor (PRR) responsive to mannose/chitin (mrc1) and a gene involved A.M.; Ramalho, L.N.Z.; Almeida, F.