Anti-Inflammatory Activity of Igg1 Mediated by Fc Galactosylation And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Ligands for Human Igg and Their Effector Functions
antibodies Review The Ligands for Human IgG and Their Effector Functions Steven W. de Taeye 1,2,*, Theo Rispens 1 and Gestur Vidarsson 2 1 Sanquin Research, Dept Immunopathology and Landsteiner Laboratory, Amsterdam UMC, University of Amsterdam, 1066 CX Amsterdam, The Netherlands; [email protected] 2 Sanquin Research, Dept Experimental Immunohematology and Landsteiner Laboratory, Amsterdam UMC, University of Amsterdam, 1066 CX Amsterdam, The Netherlands; [email protected] * Correspondence: [email protected] Received: 26 March 2019; Accepted: 18 April 2019; Published: 25 April 2019 Abstract: Activation of the humoral immune system is initiated when antibodies recognize an antigen and trigger effector functions through the interaction with Fc engaging molecules. The most abundant immunoglobulin isotype in serum is Immunoglobulin G (IgG), which is involved in many humoral immune responses, strongly interacting with effector molecules. The IgG subclass, allotype, and glycosylation pattern, among other factors, determine the interaction strength of the IgG-Fc domain with these Fc engaging molecules, and thereby the potential strength of their effector potential. The molecules responsible for the effector phase include the classical IgG-Fc receptors (FcγR), the neonatal Fc-receptor (FcRn), the Tripartite motif-containing protein 21 (TRIM21), the first component of the classical complement cascade (C1), and possibly, the Fc-receptor-like receptors (FcRL4/5). Here we provide an overview of the interactions of IgG with effector molecules and discuss how natural variation on the antibody and effector molecule side shapes the biological activities of antibodies. The increasing knowledge on the Fc-mediated effector functions of antibodies drives the development of better therapeutic antibodies for cancer immunotherapy or treatment of autoimmune diseases. -

Propranolol-Mediated Attenuation of MMP-9 Excretion in Infants with Hemangiomas
Supplementary Online Content Thaivalappil S, Bauman N, Saieg A, Movius E, Brown KJ, Preciado D. Propranolol-mediated attenuation of MMP-9 excretion in infants with hemangiomas. JAMA Otolaryngol Head Neck Surg. doi:10.1001/jamaoto.2013.4773 eTable. List of All of the Proteins Identified by Proteomics This supplementary material has been provided by the authors to give readers additional information about their work. © 2013 American Medical Association. All rights reserved. Downloaded From: https://jamanetwork.com/ on 10/01/2021 eTable. List of All of the Proteins Identified by Proteomics Protein Name Prop 12 mo/4 Pred 12 mo/4 Δ Prop to Pred mo mo Myeloperoxidase OS=Homo sapiens GN=MPO 26.00 143.00 ‐117.00 Lactotransferrin OS=Homo sapiens GN=LTF 114.00 205.50 ‐91.50 Matrix metalloproteinase‐9 OS=Homo sapiens GN=MMP9 5.00 36.00 ‐31.00 Neutrophil elastase OS=Homo sapiens GN=ELANE 24.00 48.00 ‐24.00 Bleomycin hydrolase OS=Homo sapiens GN=BLMH 3.00 25.00 ‐22.00 CAP7_HUMAN Azurocidin OS=Homo sapiens GN=AZU1 PE=1 SV=3 4.00 26.00 ‐22.00 S10A8_HUMAN Protein S100‐A8 OS=Homo sapiens GN=S100A8 PE=1 14.67 30.50 ‐15.83 SV=1 IL1F9_HUMAN Interleukin‐1 family member 9 OS=Homo sapiens 1.00 15.00 ‐14.00 GN=IL1F9 PE=1 SV=1 MUC5B_HUMAN Mucin‐5B OS=Homo sapiens GN=MUC5B PE=1 SV=3 2.00 14.00 ‐12.00 MUC4_HUMAN Mucin‐4 OS=Homo sapiens GN=MUC4 PE=1 SV=3 1.00 12.00 ‐11.00 HRG_HUMAN Histidine‐rich glycoprotein OS=Homo sapiens GN=HRG 1.00 12.00 ‐11.00 PE=1 SV=1 TKT_HUMAN Transketolase OS=Homo sapiens GN=TKT PE=1 SV=3 17.00 28.00 ‐11.00 CATG_HUMAN Cathepsin G OS=Homo -

Microglial Function and Regulation During Development, Homeostasis and Alzheimer’S Disease
cells Review Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease Brad T. Casali and Erin G. Reed-Geaghan * Department of Pharmaceutical Sciences, Northeast Ohio Medical University, Rootstown, OH 44272, USA; [email protected] * Correspondence: [email protected] Abstract: Microglia are the resident immune cells of the brain, deriving from yolk sac progenitors that populate the brain parenchyma during development. During development and homeostasis, microglia play critical roles in synaptogenesis and synaptic plasticity, in addition to their primary role as immune sentinels. In aging and neurodegenerative diseases generally, and Alzheimer’s disease (AD) specifically, microglial function is altered in ways that significantly diverge from their homeostatic state, inducing a more detrimental inflammatory environment. In this review, we discuss the receptors, signaling, regulation and gene expression patterns of microglia that mediate their phenotype and function contributing to the inflammatory milieu of the AD brain, as well as strategies that target microglia to ameliorate the onset, progression and symptoms of AD. Keywords: microglia; inflammation; Alzheimer’s disease; neurodegenerative diseases; TREM2; neu- roinflammation Citation: Casali, B.T.; Reed-Geaghan, 1. Introduction E.G. Microglial Function and Microglia are the resident phagocytes of the central nervous system (CNS). In addition Regulation during Development, to their immunological role in maintaining CNS homeostasis, microglia play vital -

Assessing the Tumor Microenvironment by Recovery of Immune Receptor V(D)J Recombination Reads from Tumor Specimen Exome Files
Assessing the Tumor Microenvironment by Recovery of Immune Receptor V(D)J Recombination Reads from Tumor Specimen Exome Files George Blanck, Ph.D. Professor, Molecular Medicine Morsani College of Medicine, USF Immunology Program, Moffitt Cancer Center (not for publication or public web page) Learning objectives: 1. To appreciate the availability of T-cell receptor recombination information from tumor specimen DNA samples. 2. To understand the correlation between T-cell receptor recombinations, in tumor specimen DNA, and other, clinically relevant information for bladder cancer and kidney renal cell carcinoma. 3. To understand the importance of HLA alleles in assessing the impact of T-cell receptor recombinations from tumor specimen DNA. Fig. 1. Immune receptor genes recombine during development to generate many different receptor molecules among the B-cells and T- cells, throughout the body, through life, to bind many different antigens, including tumor antigens. Seven immune receptor genes total: • Three related to antibodies, not further discussed. • Two related to gamma-delta T-cells, not further discussed • Two required for alpha-beta T-cells, the subject of this presentation. Alpha-beta T-cells: • Most numerous. • Best understood, medically speaking. • Generally target peptide antigen, in the case of tumors, a mutant peptide. • The TRB part of the TRA/TRB receptor is considered most important in antigen binding. The tumor specimen and the exome • Surgically remove tumor, obtain DNA sequence (all exons = exome), for tumor mutations, which can guide therapy. • Other cells in the specimen, particularly T-cells. • The DNA representing the T-cell receptor can be identified above the tumor DNA “background”. Fig. -

Transcriptional Control of Tissue-Resident Memory T Cell Generation
Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2019 © 2019 Filip Cvetkovski All rights reserved ABSTRACT Transcriptional control of tissue-resident memory T cell generation Filip Cvetkovski Tissue-resident memory T cells (TRM) are a non-circulating subset of memory that are maintained at sites of pathogen entry and mediate optimal protection against reinfection. Lung TRM can be generated in response to respiratory infection or vaccination, however, the molecular pathways involved in CD4+TRM establishment have not been defined. Here, we performed transcriptional profiling of influenza-specific lung CD4+TRM following influenza infection to identify pathways implicated in CD4+TRM generation and homeostasis. Lung CD4+TRM displayed a unique transcriptional profile distinct from spleen memory, including up-regulation of a gene network induced by the transcription factor IRF4, a known regulator of effector T cell differentiation. In addition, the gene expression profile of lung CD4+TRM was enriched in gene sets previously described in tissue-resident regulatory T cells. Up-regulation of immunomodulatory molecules such as CTLA-4, PD-1, and ICOS, suggested a potential regulatory role for CD4+TRM in tissues. Using loss-of-function genetic experiments in mice, we demonstrate that IRF4 is required for the generation of lung-localized pathogen-specific effector CD4+T cells during acute influenza infection. Influenza-specific IRF4−/− T cells failed to fully express CD44, and maintained high levels of CD62L compared to wild type, suggesting a defect in complete differentiation into lung-tropic effector T cells. -

FCGR2B) Is Associated with the Production of Anti-Cyclic Citrullinated Peptide Autoantibodies in Taiwanese RA
Genes and Immunity (2008) 9, 680–688 & 2008 Macmillan Publishers Limited All rights reserved 1466-4879/08 $32.00 www.nature.com/gene ORIGINAL ARTICLE A transmembrane polymorphism in FcgRIIb (FCGR2B) is associated with the production of anti-cyclic citrullinated peptide autoantibodies in Taiwanese RA J-Y Chen1, C-M Wang2, C-C Ma1, L-A Hsu3, H-H Ho1, Y-JJ Wu1, S-N Kuo1 and J Wu4 1Division of Allergy, Immunology and Rheumatology, Department of Medicine, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Tao-Yuan, Taiwan, Republic of China; 2Department of Rehabilitation, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Tao-Yuan, Taiwan, Republic of China; 3Department of Medicine, Division of First Cardiovascular, Chang Gung Memorial Hospital, Chang Gung University College of Medicine, Tao-Yuan, Taiwan, Republic of China and 4Division of Clinical Immunology and Rheumatology, Department of Medicine, University of Alabama at Birmingham, Birmingham, AL, USA The aim of the current study was to determine whether the FcgRIIb 187-Ile/Thr polymorphism is a predisposition factor for subtypes of RA defined by disease severity and production of autoantibodies against cyclic citrullinated peptides (anti-CCPs) in Taiwanese RA patients. Genotype distributions and allele frequencies of FcgRIIb 187-Ile/Thr were compared between 562 normal healthy controls and 640 RA patients as stratified by clinical parameters and autoantibodies. Significant enrichment of 187-Ile allele was observed in RA patients positive for anti-CCP antibodies as compared with the anti-CCP negative RA patients (P ¼ 0.001, OR 1.652 (95% CI 1.210–2.257)) or as compared with the normal controls (P ¼ 0.005, OR 1.348 (95% CI 1.092–1.664)). -

IL-25 and Type 2 Innate Lymphoid Cells Induce Pulmonary Fibrosis
IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis Emily Hamsa,b, Michelle E. Armstronga,b, Jillian L. Barlowc, Sean P. Saundersa,b, Christian Schwartzd, Gordon Cookee, Ruairi J. Fahyf, Thomas B. Crottyg, Nikhil Hiranih, Robin J. Flynnc, David Voehringerd, Andrew N. J. McKenziec, Seamas C. Donnellye,i, and Padraic G. Fallona,b,j,1 aTrinity Biomedical Sciences Institute, School of Medicine, Trinity College Dublin, Dublin 2, Ireland; bNational Children’s Research Centre, Our Lady’s Children’s Hospital, Dublin 8, Ireland; cMedical Research Council Laboratory of Molecular Biology, Cambridge CB2 0QH, United Kingdom; dDepartment of Infection Biology, Universitätsklinikum Erlangen, 91054 Erlangen, Germany; eSchool of Medicine and Medical Science, University College Dublin, Dublin 4, Ireland; fSt. James’s Hospital, Dublin 8, Ireland; gDepartment of Pathology, St. Vincent’s University Hospital, Dublin 4, Ireland; hMedical Research Council Centre for Inflammation Research, University of Edinburgh, Edinburgh EH16 4SB, United Kingdom; iNational Pulmonary Fibrosis Referral Centre, St. Vincent’s University Hospital, Dublin 4, Ireland; and jInstitute of Molecular Medicine, School of Medicine, Trinity College Dublin, St. James’s Hospital, Dublin 8, Ireland Edited by Shigeo Koyasu, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan, and accepted by the Editorial Board November 15, 2013 (received for review August 26, 2013) Disease conditions associated with pulmonary fibrosis are pro- fibrosis in experimental mouse models, via the activation of IL-13– gressive and have a poor long-term prognosis with irreversible producing ILC2. We also observe increases in both IL-25 and ILC2 changes in airway architecture leading to marked morbidity and in the lung of patients with idiopathic pulmonary fibrosis (IPF). -
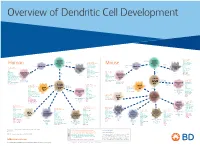
View Dendritic Cell Development Poster
Overview of Dendritic Cell Development Lineage–, CD45+, Common CD117 (c-kit) Common MHCII+, CD11c+ – + CD207 (Langerin) Myeloid CD117 (c-kit) Lineage , CD45 , Myeloid Progenitor MHCII (HLA-DR)+, CD11c+ Progenitor CD324 (E-Cadherin) Human Mouse CD326 (EpCAM) CD207 (Langerin) TGFb1 Cells CD11b, CD115 Cells CD14 Monocyte CD324 (E-Cadherin) Monocyte M-CSF CD11b – + Ly6C Langerhans CD24 Lineage , CD45 , M-CSF CD326 (EpCAM) MHCII (HLA-DR)+, CD11c+ Langerhans CD11blo Zbtb46– Cells CD172a (Sirp-α) CD16 CD1ahi, CD1c CD205 (DEC-205) Cells CSF F4/80 CD64 CD172a (Sirp-α) Lineage–, CD45+, FLT3L TLR3, TLR11 CD1a, CD1c Inflammatory CD369 (Dectin-1/CLEC7A) MHCII+, CD11c+ +/– CSF IL-15 CD8–, CD14– CD11b, CD14 CD371 (CLEC12A) CD64 Monocyte- FLT3L Inflammatory CD370 (Clec9a)– CD172a (Sirp-α) IL-15 CLEC6A CD11b derived lo Monocyte- CD206, CD209 (DC-SIGN) TLR1, TLR2, TLR3 , TLR6 CD209a (DC-SIGN) CD367 (DCIR/CLEC4A) DCs CD14– CD272 (BTLA)lo derived CD369 (Dectin-1/CLEC7A) DCs Common Ly-6C – + CD371 (CLEC12A) CD117 (c-kit) Lineage , CD45 , IL-1β, IL-6, IL-10, TLR1-6, TLR7-8, TLR10 Dendritic + lo CLEC6A – – CD135/FLT3 MHCII , CD11c IL-12, IL-23, TNF CD8a , CD14 IL-1β, IL-6 IL-10, Precursor TLR3lo, TLR4, TLR7, TLR8 CD45R (B220) IL-12, IL-23, TNF Plasmacytoid CD207 (Langerin)– Cells CD317 (BST-2) Common Lineage–, CD45+, FLT3L DCs Lineage–, CD45+, + Ly6C + lo/– CD207 IFN Type I + + Dendritic CD135/FLT3 MHCII (HLA-DR) , CD11c Lineage–, CD45+, IRF7, IRF8, BATF3hi Siglec-H MHCII (HLA-DR) , CD11c hi – + CD123 + + Dermal SpiB, Zbtb46 CD1a, CD64 CD1a Precursor CD117 (c-kit) -

Supplementary Table 1: Adhesion Genes Data Set
Supplementary Table 1: Adhesion genes data set PROBE Entrez Gene ID Celera Gene ID Gene_Symbol Gene_Name 160832 1 hCG201364.3 A1BG alpha-1-B glycoprotein 223658 1 hCG201364.3 A1BG alpha-1-B glycoprotein 212988 102 hCG40040.3 ADAM10 ADAM metallopeptidase domain 10 133411 4185 hCG28232.2 ADAM11 ADAM metallopeptidase domain 11 110695 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 195222 8038 hCG40937.4 ADAM12 ADAM metallopeptidase domain 12 (meltrin alpha) 165344 8751 hCG20021.3 ADAM15 ADAM metallopeptidase domain 15 (metargidin) 189065 6868 null ADAM17 ADAM metallopeptidase domain 17 (tumor necrosis factor, alpha, converting enzyme) 108119 8728 hCG15398.4 ADAM19 ADAM metallopeptidase domain 19 (meltrin beta) 117763 8748 hCG20675.3 ADAM20 ADAM metallopeptidase domain 20 126448 8747 hCG1785634.2 ADAM21 ADAM metallopeptidase domain 21 208981 8747 hCG1785634.2|hCG2042897 ADAM21 ADAM metallopeptidase domain 21 180903 53616 hCG17212.4 ADAM22 ADAM metallopeptidase domain 22 177272 8745 hCG1811623.1 ADAM23 ADAM metallopeptidase domain 23 102384 10863 hCG1818505.1 ADAM28 ADAM metallopeptidase domain 28 119968 11086 hCG1786734.2 ADAM29 ADAM metallopeptidase domain 29 205542 11085 hCG1997196.1 ADAM30 ADAM metallopeptidase domain 30 148417 80332 hCG39255.4 ADAM33 ADAM metallopeptidase domain 33 140492 8756 hCG1789002.2 ADAM7 ADAM metallopeptidase domain 7 122603 101 hCG1816947.1 ADAM8 ADAM metallopeptidase domain 8 183965 8754 hCG1996391 ADAM9 ADAM metallopeptidase domain 9 (meltrin gamma) 129974 27299 hCG15447.3 ADAMDEC1 ADAM-like, -

Identification of Immunologic and Genetic Biomarkers for TMA Sensitization
Identification of Immunologic and genetic biomarkers for TMA sensitization Debajyoti Ghosh PhD Research Scientist Internal Medicine, Division of Immunology University of Cincinnati College of Medicine Funding, lab support and mentorship • Funding: Supported by the National Institute for Occupational Safety and Health Pilot Research Project Training Program of the University of Cincinnati Education and Research Center Grant #T42/0H008432-09. • Lab-support and mentorship: Dr. Jonathan Bernstein MD (lab director) Department of Internal Medicine, UC College of Medicine Dr. Ian Lewkowich PhD Div. of Immunobiology Cincinnati Children’s Hospital and Medical Center Trimellitic Anhydride (TMA) • Hardening agent used in preparing plastics and paints. • 100,000 metric tons produced annually worldwide (65,000 metric tons/year in the U.S.). • An estimated 20,000 workers in the US are currently TMA:C H O exposed to trimellitic anhydride. 9 4 5 MW:192.13 • Free TMA can be converted to TMLA. It is an irritant (can’t induce antibody response). • Numerous TMA molecules can irreversibly bind to a large carrier protein (e.g. HSA) to make a complete antigen. This can cause allergenic antibody responses leading to occupational asthma. TMA TMA-HSA • TMA is a unique molecule responsible for producing both irritant and allergnic responses. Ref. http://www.inchem.org/documents (accessed Oct., 2015) http://www.cdc.gov/niosh/docs/1970/78121_21.html (accessed Oct, 2015) Irritant versus allergenic response • A reversible inflammatory effect on • Usually caused by antibody- living tissue by chemical action at the mediated systemic reactions. IgE is site of contact. Not antibody-mediated. the key molecule. • Non-specific reaction, can be caused • Very specific, mediated by Ag-Ab by any irritant. -

A New Role for NKG2D Signaling in CD8 T Cells and Autoimmune
A new role for NKG2D signaling in CD8+ T cells and autoimmune diabetes By Andrew P Trembath © 2019 Submitted to the graduate degree program in Microbiology, Molecular Genetics and Immunology, and the Graduate Faculty of the University of Kansas in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Chair: Mary A. Markiewicz Ph.D Wolfram R. Zückert Ph.D. Patrick E. Fields, Ph.D. Maria Kalamvoki Ph.D. Joe Lutkenhaus Ph.D Date Defended: 11/13/2019 ii The dissertation committee for Andrew Trembath certifies that this is the approved version of the following dissertation: A new role for NKG2D signaling in CD8+ T cells and autoimmune diabetes Chair: Mary A. Markiewicz, Ph.D. Date Approved: 12/19/2019 iii Abstract The demands placed on the immune system are immense and highly complex. It must protect the body against untold threats while maintaining a balance between immune defense and autoimmune damage. One major player in immune recognition is the receptor Natural-Killer- Group-2-Member-D (NKG2D), best known for its expression on natural killer (NK) cells and CD8+ T cells, where it recognizes NKG2D ligands expressed by stressed cells following viral infection or cancerous transformation. NKG2D is most well studied for its role in tumor immunity, for which NKG2D based therapies are currently being developed clinically. Despite this, it is apparent that NKG2D has other poorly understood immune regulating functions, such as its implicated involvement in type 1diabetes and other autoimmune disorders. However, the mechanism by which NKG2D signaling affects diabetes has been unclear. We therefore sought to further clarify the role NKG2D plays in autoimmune diabetes development. -

Toll and Toll-Like Receptor Signalling in Development Niki Anthoney*, Istvan Foldi‡ and Alicia Hidalgo§
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Repository of the Academy's Library © 2018. Published by The Company of Biologists Ltd | Development (2018) 145, dev156018. doi:10.1242/dev.156018 DEVELOPMENT AT A GLANCE Toll and Toll-like receptor signalling in development Niki Anthoney*, Istvan Foldi‡ and Alicia Hidalgo§ ABSTRACT signalling and discuss how this signalling pathway regulates various The membrane receptor Toll and the related Toll-like receptors aspects of development across species. (TLRs) are best known for their universal function in innate immunity. KEY WORDS: Toll, Tol-1, TLR, TIR-NBS-LRR, Sarm, MyD88, NF-κB, However, Toll/TLRs were initially discovered in a developmental Dorsal, Wek, JNK, FoxO, Cell death, Cell survival, Cell fate, context, and recent studies have revealed that Toll/TLRs carry out Cell proliferation, Structural plasticity, Signalling previously unanticipated functions in development, regulating cell fate, cell number, neural circuit connectivity and synaptogenesis. Introduction Furthermore, knowledge of their molecular mechanisms of action is The Drosophila gene Toll is possibly the only gene associated with expanding and has highlighted that Toll/TLRs function beyond the two Nobel Prizes: it was discovered as one of the key genes canonical NF-κB pathway to regulate cell-to-cell communication and determining body plan, and was later rediscovered for its role in signalling at the synapse. Here, we provide an overview of Toll/TLR underlying innate immunity (leading to the 1995 and 2011 Nobel Prizes in Physiology or Medicine, respectively). Searches for Toll NeuroDevelopment Group, School of Biosciences, University of Birmingham, homologues led to the identification of Toll-like receptor (TLR) Birmingham B15 2TT, UK.