Regeneration Status of Tree Species with Different Functional Traits in Tropical Forest Remnants a Case Study of Crater Forests in Western Uganda
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The 140 Bird Species Recorded in the Korsman Conservancy
The 140 Bird Species Recorded in the Korsman Conservancy Roberts 6 Scientific Name Probability Residence Season Alphabetical Name Full Name 294 Avocet, Pied Pied Avocet Recurvirostra avosetta 11% 1 All Year 464 Barbet, Black-collared Black-collared Barbet Lybius torquatus 56% 1 All Year 473 Barbet, Crested Crested Barbet Trachyphonus vaillantii 83% 1 All Year 824 Bishop, Southern Red Southern Red Bishop Euplectes orix 73% 1 All Year 826 Bishop, Yellow-crowned Yellow-crowned Bishop Euplectes afer 14% 1 All Year 78 Bittern, Little Little Bittern Ixobrychus minutus 7% 3.1 Dec-March 568 Bulbul, Dark-capped Dark-capped Bulbul Pycnonotus tricolor 86% 1 All Year 149 Buzzard, Common (Steppe ) Common (Steppe) Buzzard Buteo buteo 1% 3.1 Oct-Apr 130 Buzzard, European Honey European Honey Buzzard Pernis apivorus 3.1 Nov-Apr 870 Canary, Black-throated Black-throated Canary Crithagra atrogularis 11% 1 All Year 677 Cisticola, Levaillant’s Levaillant’s Cisticola Cisticola tinniens 36% 1 All Year 664 Cisticola, Zitting Zitting Cisticola Cisticola juncidis 13% 1 All Year 228 Coot, Red-knobbed Red-knobbed coot Fulica cristata 90% 1 All Year 58 Cormorant, Reed Reed Cormorant Phalacrocorax africanus 77% 1 All Year 55 Cormorant, White-breasted White-breasted Cormorant Phalacrocorax lucidus 62% 1 All Year 391 Coucal, Burchell’s Burchell’s Coucal Centropus burchellii 13% 1 All Year 213 Crake, Black Black Crake Amaurornis flavirostra 7% 1 All Year 548 Crow, Pied Pied crow Corvus albus 30% 1 All Year 386 Cuckoo, Diederik Diederik Cuckoo Chrysococcyx caprius 15% -

TNP SOK 2011 Internet
GARDEN ROUTE NATIONAL PARK : THE TSITSIKAMMA SANP ARKS SECTION STATE OF KNOWLEDGE Contributors: N. Hanekom 1, R.M. Randall 1, D. Bower, A. Riley 2 and N. Kruger 1 1 SANParks Scientific Services, Garden Route (Rondevlei Office), PO Box 176, Sedgefield, 6573 2 Knysna National Lakes Area, P.O. Box 314, Knysna, 6570 Most recent update: 10 May 2012 Disclaimer This report has been produced by SANParks to summarise information available on a specific conservation area. Production of the report, in either hard copy or electronic format, does not signify that: the referenced information necessarily reflect the views and policies of SANParks; the referenced information is either correct or accurate; SANParks retains copies of the referenced documents; SANParks will provide second parties with copies of the referenced documents. This standpoint has the premise that (i) reproduction of copywrited material is illegal, (ii) copying of unpublished reports and data produced by an external scientist without the author’s permission is unethical, and (iii) dissemination of unreviewed data or draft documentation is potentially misleading and hence illogical. This report should be cited as: Hanekom N., Randall R.M., Bower, D., Riley, A. & Kruger, N. 2012. Garden Route National Park: The Tsitsikamma Section – State of Knowledge. South African National Parks. TABLE OF CONTENTS 1. INTRODUCTION ...............................................................................................................2 2. ACCOUNT OF AREA........................................................................................................2 -

Plant-Frugivore Interactions in a Heterogeneous Forest Landscape of South Africa
Plant-frugivore interactions in a heterogeneous forest landscape of South Africa Dissertation In partial fulfilment of the requirements for the award of a Doctorate Degree in Natural Sciences (Dr. rer. nat) The Faculty of Biology, Philipps-University of Marburg Lackson Chama, MSc Sinazongwe (Zambia) June 2012, Marburg From the Faculty of Biology, Philipps-University Marburg als Dissertation am angenommen. Dekan: Prof. Dr. Paul Galland Erstgutachterin: Prof. Dr. N. Farwig Zweitgutachter: Prof. Dr. R. Brandl Tag der Disputation: 25th June 2012 Dedicated to my son, Mishila, who’s first two years on earth I was hardly part of, due to my commitment towards this work. Contents CHAPTER 1: GENERAL INTRODUCTION ..................................................................................................................... 3 EFFECTS OF HUMAN ACTIVITIES ON FOREST BIODIVERSITY ........................................................................................................ 4 PLANT-FRUGIVORE INTERACTIONS IN CHANGING LANDSCAPES .................................................................................................. 5 THE ROLE OF FUNCTIONAL DIVERSITY IN FRUGIVORE COMMUNITIES ........................................................................................... 5 EFFECTS OF SEED INGESTION BY FRUGIVOROUS BIRDS ON GERMINATION SUCCESS ........................................................................ 6 AIMS OF THE THESIS ......................................................................................................................................................... -

Birds of the Agulhas Plain
Birds of the Agulhas Plain www.blackoystercatcher.co.za Birds of the Agulhas Plain – how to see them “Whether you love bird-watching or not, a trip to the Black Oystercatcher will always include a vast array of bird life en route.” www.blackoystercatcher.co.za The Black Oystercatcher Wine farm is based on (and is a member of) the Nuwejaars Wetland Special Management Area. It’s a conservation agricultural area home to some of the most endangered bird, plant and animal species in the world. That makes the region perfect for bird lovers – whether you’re an experienced bird-watcher, or are just starting to record your sightings for the first time. So if you’re new to bird-watching, or new to the area, here are a couple of tips to record bird species you may not have seen before: REMEMBER TO BRING YOU BINOCULARS. Loads of DON’T ASSUME ANYTHING. You may think the raptor birds sit along the fence posts on the journey to the sitting on the fence is a Common Buzzard. But don’t Black Oystercatcher. But they usually don’t hang discount the possibility that it may be something more around if you stop. So a pair of binoculars will help you exotic and more rare. Some of the bird species spotted on check their colour, shape and size, even when they take or close to the Black Oystercatcher include the Long to the skies. Crested Eagle, Montagu’s Harrier and Blue-cheeked Bee- HAVE YOUR BIRD GUIDE READY. Whether it be Sasol, eater – species not known to frequent the Agulhas Plain. -

Manyoni Private Game Reserve (Previously Zululand Rhino Reserve)
Manyoni Private Game Reserve (Previously Zululand Rhino Reserve) Gorgeous Bushshrike by Adam Riley BIRD LIST Prepared by Adam Riley [email protected] • www.rockjumperbirding. -

Protected Area Management Plan Development - SAPO NATIONAL PARK
Technical Assistance Report Protected Area Management Plan Development - SAPO NATIONAL PARK - Sapo National Park -Vision Statement By the year 2010, a fully restored biodiversity, and well-maintained, properly managed Sapo National Park, with increased public understanding and acceptance, and improved quality of life in communities surrounding the Park. A Cooperative Accomplishment of USDA Forest Service, Forestry Development Authority and Conservation International Steve Anderson and Dennis Gordon- USDA Forest Service May 29, 2005 to June 17, 2005 - 1 - USDA Forest Service, Forestry Development Authority and Conservation International Protected Area Development Management Plan Development Technical Assistance Report Steve Anderson and Dennis Gordon 17 June 2005 Goal Provide support to the FDA, CI and FFI to review and update the Sapo NP management plan, establish a management plan template, develop a program of activities for implementing the plan, and train FDA staff in developing future management plans. Summary Week 1 – Arrived in Monrovia on 29 May and met with Forestry Development Authority (FDA) staff and our two counterpart hosts, Theo Freeman and Morris Kamara, heads of the Wildlife Conservation and Protected Area Management and Protected Area Management respectively. We decided to concentrate on the immediate implementation needs for Sapo NP rather than a revision of existing management plan. The four of us, along with Tyler Christie of Conservation International (CI), worked in the CI office on the following topics: FDA Immediate -

Status and Distribution of Faunal Diversity in Kafa Afromontane Coffee Forest
Status and Distribution of Faunal Diversity in Kafa Afromontane Coffee Forest Leykun Abunie Berhan Submitted to PPP Project July 2008 Addis Ababa Contents Executive Summary .....................................................................................................................4 Introduction..................................................................................................................................6 Literature Review Related to Faunal Diversity and Management...............................................8 Macro Policies and Priorities......................................................................................................8 Environmental Protection Policy.................................................................................................8 Wildlife Development / Management Policy................................................................................9 Analysis of Wildlife Sector in Ethiopia ......................................................................................10 Physical and Ecological Description of the Study Area ............................................................14 Objective of the Present Study...................................................................................................16 Methodology ..............................................................................................................................17 General Approach......................................................................................................................17 -

Title of Thesis Or Dissertation, Worded
HUMAN-WILDLIFE CONFLICT AND ECOTOURISM: COMPARING PONGARA AND IVINDO NATIONAL PARKS IN GABON by SANDY STEVEN AVOMO NDONG A THESIS Presented to the Department of International Studies and the Graduate School of the University of Oregon in partial fulfillment of the requirements for the degree of Master of Arts September 2017 THESIS APPROVAL PAGE Student: Sandy Steven Avomo Ndong Title: Human-wildlife Conflict: Comparing Pongara and Ivindo National Parks in Gabon This thesis has been accepted and approved in partial fulfillment of the requirements for the Master of Arts degree in the Department of International Studies by: Galen Martin Chairperson Angela Montague Member Derrick Hindery Member and Sara D. Hodges Interim Vice Provost and Dean of the Graduate School Original approval signatures are on file with the University of Oregon Graduate School. Degree awarded September 2017 ii © 2017 Sandy Steven Avomo Ndong iii THESIS ABSTRACT Sandy Steven Avomo Ndong Master of Arts Department of International Studies September 2017 Title: Human-wildlife Conflict: Comparing Pongara and Ivindo National Parks in Gabon Human-wildlife conflicts around protected areas are important issues affecting conservation, especially in Africa. In Gabon, this conflict revolves around crop-raiding by protected wildlife, especially elephants. Elephants’ crop-raiding threaten livelihoods and undermines conservation efforts. Gabon is currently using monetary compensation and electric fences to address this human-elephant conflict. This thesis compares the impacts of the human-elephant conflict in Pongara and Ivindo National Parks based on their idiosyncrasy. Information was gathered through systematic review of available literature and publications, observation, and semi-structured face to face interviews with local residents, park employees, and experts from the National Park Agency. -
The Collection of Birds from São Tomé and Príncipe at the Instituto De Investigação Científica Tropical of the University of Lisbon (Portugal)
A peer-reviewed open-access journal ZooKeys 600:The 155–167 collection (2016) of birds from São Tomé and Príncipe at the Instituto de Investigação... 155 doi: 10.3897/zookeys.600.7899 DATA PAPER http://zookeys.pensoft.net Launched to accelerate biodiversity research The collection of birds from São Tomé and Príncipe at the Instituto de Investigação Científica Tropical of the University of Lisbon (Portugal) Miguel Monteiro1,2, Luís Reino1,2,3, Martim Melo1,4, Pedro Beja1,2, Cristiane Bastos-Silveira5, Manuela Ramos7, Diana Rodrigues5, Isabel Queirós Neves5,6, Susana Consciência8, Rui Figueira1,2 1 CIBIO/InBIO-Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade do Porto, Vairão, Portugal 2 CEABN/InBio, Centro de Ecologia Aplicada “Professor Baeta Neves”, Instituto Superior de Agronomia, Universidade de Lisboa, Tapada da Ajuda, 1349-017 Lisboa, Portugal 3 CIBIO/InBIO-Centro de Investigação em Biodiversidade e Recursos Genéticos, Universidade de Évora, 7004-516 Évora, Portugal 4 Percy FitzPatrick Institute of African Ornithology, University of Cape Town, Rondebosch 7701X, South Africas 5 Museu Nacional de História Natural e da Ciência, Universidade de Lisboa, Rua da Escola Politécnica 56, 1250-102 Lisboa, Portugal 6 CESAM-Centre for Environmental and Marine Studies, Universidade de Aveiro, 3810-193 Aveiro, Portugal 7 MARE-FCUL, DOP/UAç - Departamento Oceanografia e Pescas, Univ. Açores, Rua Prof. Dr. Fre- derico Machado, 9901-862 Horta, Portugal 8 Estrada de Mem Martins n251 1ºDto, 2725-391 Mem Martins, Sintra, Portugal Corresponding author: Rui Figueira ([email protected]) Academic editor: G. Sangster | Received 29 January 2015 | Accepted 2 June 2016 | Published 22 June 2016 http://zoobank.org/68209E54-00D0-4EFA-B095-AB7D346ACD8E Citation: Monteiro M, Reino L, Melo M, Beja P, Bastos-Silveira C, Ramos M, Rodrigues D, Neves IQ, Consciência S, Figueira R (2016) The collection of birds from São Tomé and Príncipe at the Instituto de Investigação Científica Tropical of the University of Lisbon (Portugal). -
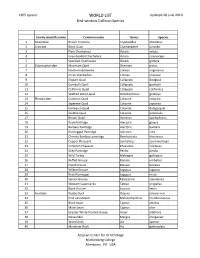
WORLD LIST Updated 28 June 2019 Bird-Window Collision Species
1305 species WORLD LIST Updated 28 June 2019 Bird-window Collision Species Family scientific name Common name Genus Species 1 Tinamidae Brown Tinamou Crypturellus obsoletus 2 Cracidae Black Guan Chamaepetes unicolor 3 Plain Chachalaca Ortalis vetula 4 Grey-headed Chachalaca Ortalis cinereiceps 5 Speckled Chachalaca Ortalis guttata 6 Odontophoridae Mountain Quail Oreortyx pictus 7 Northern Bobwhite Colinus virginianus 8 Crested Bobwhite Colinus cristatus 9 Elegant Quail Callipepla douglasii 10 Gambel's Quail Callipepla gambelii 11 California Quail Callipepla californica 12 Spotted Wood-quail Odontophorus guttatus 13 Phasianidae Common Quail Coturnix coturnix 14 Japanese Quail Coturnix japonica 15 Harlequin Quail Coturnix delegorguei 16 Stubble Quail Coturnix pectoralis 17 Brown Quail Synoicus ypsilophorus 18 Rock Partridge Alectoris graeca 19 Barbary Partridge Alectoris barbara 20 Red-legged Partridge Alectoris rufa 21 Chinese Bamboo-partridge Bambusicola thoracicus 22 Copper Pheasant Syrmaticus soemmerringii 23 Common Pheasant Phasianus colchicus 24 Grey Partridge Perdix perdix 25 Wild Turkey Meleagris gallopavo 26 Ruffed Grouse Bonasa umbellus 27 Hazel Grouse Bonasa bonasia 28 Willow Grouse Lagopus lagopus 29 Rock Ptarmigan Lagopus muta 30 Spruce Grouse Falcipennis canadensis 31 Western Capercaillie Tetrao urogallus 32 Black Grouse Lyrurus tetrix 33 Anatidae Ruddy Duck Oxyura jamaicensis 34 Pink-eared Duck Malacorhynchus membranaceus 35 Black Swan Cygnus atratus 36 Mute Swan Cygnus olor 37 Greater White-fronted Goose Anser albifrons 38 -

Gumede Silindile Thobeka 2020
Taxonomic, functional, and avian community dynamics in selected Southern Mistbelt Forests of southern KwaZulu-Natal and the Eastern Cape, South Africa Silindile T. Gumede Submitted in fulfilment of the academic requirements for the degree of Doctorate of Philosophy in the discipline of Ecological Sciences School of Life Sciences College of Agriculture, Engineering and Science University of KwaZulu-Natal Pietermaritzburg Campus. 2020 ABSTRACT Human population increase and landscape transformation result in the reduction of natural habitats, such as forests, causing changes in forest patch composition, habitat amount, patch size, isolation, shape, and edges. This is challenging specialist such as Orange Ground-thrush (Geokichla gurneyi) and Lemon Dove (Aplopelia larvata) species unable to survive in fragmented habitats and also influencing the composition and distribution of avian species assemblages. Therefore, the main aims of this multifaceted study were to (1) assess the habitat requirements of two forest specialised bird species, the Orange Ground-thrush (Geokichla gurneyi) and the Lemon Dove (Aplopelia larvata); (2) identify if avian assemblage diversity and species functional trait diversity show consist patterning across different landscapes in a forest ecosystem; (3) determine the influence of vegetation structures on the taxonomic and functional diversity of avian forest species, and (4) modelling how to connect forest patches of higher functional diversity. In 2018-2019, we conducted a series of camera-trap surveys of 21- day periods and fixed-radius point-count surveys at 420 sites across 94 forest patches of Southern Mistbelt Forest of southern KwaZulu-Natal and Eastern Cape, South Africa, during the breeding and non-breeding seasons in conjunction with surveys of microhabitat structural covariates. -

Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report
www.fundacaoprincipe.org Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report Project Team: Frazer Sinclair, Yodiney Dos Santos, Ayres Pedronho, Aramis Andrade, Davide Dias, and Roldeney Fernandes. Report prepared by Frazer Sinclair, Fauna & Flora International, October 2019. For further information contact: [email protected], or [email protected] How to cite this document: Fundação Príncipe. (2019) Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report. Fundação Príncipe, Santo Antonio, Príncipe Island, www.fundacaoprincipe.org. Understanding the Remarkable Biodiversity of Príncipe Island – Scientific Report 1 www.fundacaoprincipe.org Summary Principe is home to a diversity of unique fauna and flora including globally threatened species such as the Critically Endangered Príncipe Thrush (Turdus xanthorhynchus) and the Vulnerable Obô Snail (Archachatina bicarinata). The implementation of conservation plans in Príncipe has previously been impeded by a lack of capacity and resources. Our project – Understanding the Remarkable Biodiversity of Prícipe Island – has built local capacity and implemented priority actions from existing conservation plans through the completion of a series of biological and social field surveys. These included an island wide survey of bird species using a point-count method; camera trap and nesting surveys to examine threats to the Príncipe Thrush; sampling of terrestrial molluscs at 10 sites across the island; & a questionnaire based survey of 14 communities to examine forest resource use and knowledge of the Obô Snail. Key findings included: An inventory of 32 bird species, with distribution models produced for 20 of these Confirmation of a March-July breeding period for the Príncipe thrush Camera trap images of non-native mammal species within the Parque Natural do Principe, including feral cats, dogs, African civets, Mona monkeys, and black rats.