False Flowers in Asteraceae
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Floral Symmetry Affects Speciation Rates in Angiosperms Risa D
Received 25 July 2003 Accepted 13 November 2003 Published online 16 February 2004 Floral symmetry affects speciation rates in angiosperms Risa D. Sargent Department of Zoology, University of British Columbia, 6270 University Boulevard, Vancouver, British Columbia V6T 1Z4, Canada ([email protected]) Despite much recent activity in the field of pollination biology, the extent to which animal pollinators drive the formation of new angiosperm species remains unresolved. One problem has been identifying floral adaptations that promote reproductive isolation. The evolution of a bilaterally symmetrical corolla restricts the direction of approach and movement of pollinators on and between flowers. Restricting pollin- ators to approaching a flower from a single direction facilitates specific placement of pollen on the pollin- ator. When coupled with pollinator constancy, precise pollen placement can increase the probability that pollen grains reach a compatible stigma. This has the potential to generate reproductive isolation between species, because mutations that cause changes in the placement of pollen on the pollinator may decrease gene flow between incipient species. I predict that animal-pollinated lineages that possess bilaterally sym- metrical flowers should have higher speciation rates than lineages possessing radially symmetrical flowers. Using sister-group comparisons I demonstrate that bilaterally symmetric lineages tend to be more species rich than their radially symmetrical sister lineages. This study supports an important role for pollinator- mediated speciation and demonstrates that floral morphology plays a key role in angiosperm speciation. Keywords: reproductive isolation; pollination; sister group comparison; zygomorphy 1. INTRODUCTION The importance of pollinator-mediated selection in angiosperms is well supported by theory (Kiester et al. -

Alphabetical Lists of the Vascular Plant Families with Their Phylogenetic
Colligo 2 (1) : 3-10 BOTANIQUE Alphabetical lists of the vascular plant families with their phylogenetic classification numbers Listes alphabétiques des familles de plantes vasculaires avec leurs numéros de classement phylogénétique FRÉDÉRIC DANET* *Mairie de Lyon, Espaces verts, Jardin botanique, Herbier, 69205 Lyon cedex 01, France - [email protected] Citation : Danet F., 2019. Alphabetical lists of the vascular plant families with their phylogenetic classification numbers. Colligo, 2(1) : 3- 10. https://perma.cc/2WFD-A2A7 KEY-WORDS Angiosperms family arrangement Summary: This paper provides, for herbarium cura- Gymnosperms Classification tors, the alphabetical lists of the recognized families Pteridophytes APG system in pteridophytes, gymnosperms and angiosperms Ferns PPG system with their phylogenetic classification numbers. Lycophytes phylogeny Herbarium MOTS-CLÉS Angiospermes rangement des familles Résumé : Cet article produit, pour les conservateurs Gymnospermes Classification d’herbier, les listes alphabétiques des familles recon- Ptéridophytes système APG nues pour les ptéridophytes, les gymnospermes et Fougères système PPG les angiospermes avec leurs numéros de classement Lycophytes phylogénie phylogénétique. Herbier Introduction These alphabetical lists have been established for the systems of A.-L de Jussieu, A.-P. de Can- The organization of herbarium collections con- dolle, Bentham & Hooker, etc. that are still used sists in arranging the specimens logically to in the management of historical herbaria find and reclassify them easily in the appro- whose original classification is voluntarily pre- priate storage units. In the vascular plant col- served. lections, commonly used methods are systema- Recent classification systems based on molecu- tic classification, alphabetical classification, or lar phylogenies have developed, and herbaria combinations of both. -

The Origin of the Bifurcating Style in Asteraceae (Compositae)
Annals of Botany 117: 1009–1021, 2016 doi:10.1093/aob/mcw033, available online at www.aob.oxfordjournals.org The origin of the bifurcating style in Asteraceae (Compositae) Liliana Katinas1,2,*, Marcelo P. Hernandez 2, Ana M. Arambarri2 and Vicki A. Funk3 1Division Plantas Vasculares, Museo de La Plata, La Plata, Argentina, 2Laboratorio de Morfologıa Comparada de Espermatofitas (LAMCE), Facultad de Ciencias Agrarias y Forestales, Universidad Nacional de La Plata, La Plata, Argentina and 3Department of Botany, NMNH, Smithsonian Institution, Washington D.C., USA *For correspondence. E-mail [email protected] Received: 20 November 2015 Returned for revision: 22 December 2015 Accepted: 8 January 2016 Published electronically: 20 April 2016 Background and Aims The plant family Asteraceae (Compositae) exhibits remarkable morphological variation in the styles of its members. Lack of studies on the styles of the sister families to Asteraceae, Goodeniaceae and Calyceraceae, obscures our understanding of the origin and evolution of this reproductive feature in these groups. The aim of this work was to perform a comparative study of style morphology and to discuss the relevance of im- portant features in the evolution of Asteraceae and its sister families. Methods The histochemistry, venation and general morphology of the styles of members of Goodeniaceae, Calyceraceae and early branching lineages of Asteraceae were analysed and put in a phylogenetic framework to dis- cuss the relevance of style features in the evolution of these families. Key Results The location of lipophilic substances allowed differentiation of receptive from non-receptive style papillae, and the style venation in Goodeniaceae and Calyceraceae proved to be distinctive. -

Phytochemical-Constituents, Safety and Efficacy of Commonly Used Medicinal Plants for the Treatment of Malaria in Ethiopia-A Review
Pharmacy & Pharmacology International Journal Review Article Open Access Phytochemical-constituents, safety and efficacy of commonly used medicinal plants for the treatment of malaria in Ethiopia-a review Abstract Volume 7 Issue 6 - 2019 Background: Malaria is among the ten top leading causes of morbidity and mortality in children under-5 years. Due to the rise of drug-resistant parasites and limited therapeutic Tigist Abera, Rekik Ashebir, Hirut Basha, efficacy of the available drugs, there is a need to search novel antimalarial drugs from Eyob Debebe, Abiy Abebe, Asfaw Meresa, medicinal plants commonly utilized as traditional medicines. Traditional medicines Samuel Woldekidan are often more available, affordable, sometimes are perceived as more effective than Traditional and Modern Medicine Research Directorate, conventional antimalarial drugs, cultural acceptable and the relatively lower cost. Hence Ethiopian Public Health Institute, Ethiopia traditional medicine becomes the novel candidate for the search and development of drugs for the prevention and treatment of malaria. Correspondence: Tigist Abera, Traditional and Modern Medicine Research Directorate, Ethiopian Public Health Objective: The present study aimed to review phytochemical constitute, safety and efficacy Institute, Addis Ababa, Ethiopia, commonly used medicinal plants for malaria treatment in Ethiopia. Email Methods: A web-based literature search was done by using scientific databases including Received: October 07, 2019 | Published: November 26, 2019 Pub Med, Science -

Evolutionary History of Floral Key Innovations in Angiosperms Elisabeth Reyes
Evolutionary history of floral key innovations in angiosperms Elisabeth Reyes To cite this version: Elisabeth Reyes. Evolutionary history of floral key innovations in angiosperms. Botanics. Université Paris Saclay (COmUE), 2016. English. NNT : 2016SACLS489. tel-01443353 HAL Id: tel-01443353 https://tel.archives-ouvertes.fr/tel-01443353 Submitted on 23 Jan 2017 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. NNT : 2016SACLS489 THESE DE DOCTORAT DE L’UNIVERSITE PARIS-SACLAY, préparée à l’Université Paris-Sud ÉCOLE DOCTORALE N° 567 Sciences du Végétal : du Gène à l’Ecosystème Spécialité de Doctorat : Biologie Par Mme Elisabeth Reyes Evolutionary history of floral key innovations in angiosperms Thèse présentée et soutenue à Orsay, le 13 décembre 2016 : Composition du Jury : M. Ronse de Craene, Louis Directeur de recherche aux Jardins Rapporteur Botaniques Royaux d’Édimbourg M. Forest, Félix Directeur de recherche aux Jardins Rapporteur Botaniques Royaux de Kew Mme. Damerval, Catherine Directrice de recherche au Moulon Président du jury M. Lowry, Porter Curateur en chef aux Jardins Examinateur Botaniques du Missouri M. Haevermans, Thomas Maître de conférences au MNHN Examinateur Mme. Nadot, Sophie Professeur à l’Université Paris-Sud Directeur de thèse M. -

Sand Mine Near Robertson, Western Cape Province
SAND MINE NEAR ROBERTSON, WESTERN CAPE PROVINCE BOTANICAL STUDY AND ASSESSMENT Version: 1.0 Date: 06 April 2020 Authors: Gerhard Botha & Dr. Jan -Hendrik Keet PROPOSED EXPANSION OF THE SAND MINE AREA ON PORTION4 OF THE FARM ZANDBERG FONTEIN 97, SOUTH OF ROBERTSON, WESTERN CAPE PROVINCE Report Title: Botanical Study and Assessment Authors: Mr. Gerhard Botha and Dr. Jan-Hendrik Keet Project Name: Proposed expansion of the sand mine area on Portion 4 of the far Zandberg Fontein 97 south of Robertson, Western Cape Province Status of report: Version 1.0 Date: 6th April 2020 Prepared for: Greenmined Environmental Postnet Suite 62, Private Bag X15 Somerset West 7129 Cell: 082 734 5113 Email: [email protected] Prepared by Nkurenkuru Ecology and Biodiversity 3 Jock Meiring Street Park West Bloemfontein 9301 Cell: 083 412 1705 Email: gabotha11@gmail com Suggested report citation Nkurenkuru Ecology and Biodiversity, 2020. Section 102 Application (Expansion of mining footprint) and Final Basic Assessment & Environmental Management Plan for the proposed expansion of the sand mine on Portion 4 of the Farm Zandberg Fontein 97, Western Cape Province. Botanical Study and Assessment Report. Unpublished report prepared by Nkurenkuru Ecology and Biodiversity for GreenMined Environmental. Version 1.0, 6 April 2020. Proposed expansion of the zandberg sand mine April 2020 botanical STUDY AND ASSESSMENT I. DECLARATION OF CONSULTANTS INDEPENDENCE » act/ed as the independent specialist in this application; » regard the information contained in this -

Honey Bee Suite © Rusty Burlew 2015 Master Plant List by Scientific Name United States
Honey Bee Suite Master Plant List by Scientific Name United States © Rusty Burlew 2015 Scientific name Common Name Type of plant Zone Full Link for more information Abelia grandiflora Glossy abelia Shrub 6-9 http://plants.ces.ncsu.edu/plants/all/abelia-x-grandiflora/ Acacia Acacia Thorntree Tree 3-8 http://www.2020site.org/trees/acacia.html Acer circinatum Vine maple Tree 7-8 http://www.nwplants.com/business/catalog/ace_cir.html Acer macrophyllum Bigleaf maple Tree 5-9 http://treesandshrubs.about.com/od/commontrees/p/Big-Leaf-Maple-Acer-macrophyllum.htm Acer negundo L. Box elder Tree 2-10 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=a841 Acer rubrum Red maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer rubrum Swamp maple Tree 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=275374&isprofile=1&basic=Acer%20rubrum Acer saccharinum Silver maple Tree 3-9 http://en.wikipedia.org/wiki/Acer_saccharinum Acer spp. Maple Tree 3-8 http://en.wikipedia.org/wiki/Maple Achillea millefolium Yarrow Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b282 Aesclepias tuberosa Butterfly weed Perennial 3-9 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?kempercode=b490 Aesculus glabra Buckeye Tree 3-7 http://www.missouribotanicalgarden.org/PlantFinder/PlantFinderDetails.aspx?taxonid=281045&isprofile=1&basic=buckeye -
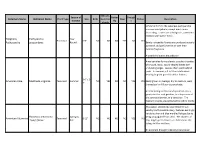
Common Name Botanical Name Alleghany
Attracts Season of Butter Drough Common Name Botanical Name Plant Type Size Birds Hummin Deer Native Description Interest fly t g birds Similar in form to the Japanese pachysandra one sees everywhere, except much more interesting. Leaves are a dull green, sometimes mottled with lighter flecks. Alleghany Pachysandra Year Perennial 6-8" NO NO NO YES NO YES Pachysandra procumbens Round Barely noticeable flowers are produced as early as March and perfume the air with their delicate fragrance. A wonderful native groundcover. American aloe forms a lovely succulent rosette of smooth, waxy, sword-shaped leaves with undulating edges. Leaves often sport reddish spots. In summer, a 3 to 5 foot stalk arises bearing fragrant greenish-white flowers. 3-6' x 2- American Aloe Manfreda virginica Perennial Summer NO YES NO NO YES YES Easily grown in average, dry to medium, well- 3' drained soil in full sun to part shade. An interesting architectural specimen, it is a good plant for rock gardens, in a dry corner of the perennial border, or a container. The fragrant blooms are pollinated by sphinx moths. This native, selected by Dale Hendrick's at nearby North Creek Nursery, features excitingly variable silver and blue marbled foliage due to Heuchera americana Spring to being propagated from seed. The clusters of American Alumroot Perennial 8-12" NO NO NO NO YES NO 'Dale's Strain' Fall tiny, bright green flowers are held above the foliage in May and June. An excellent drought tolerant groundcover. Viburnum trilobum is a native deciduous shrub to the northeastern and northwestern United States. -

Nuclear and Plastid DNA Phylogeny of the Tribe Cardueae (Compositae
1 Nuclear and plastid DNA phylogeny of the tribe Cardueae 2 (Compositae) with Hyb-Seq data: A new subtribal classification and a 3 temporal framework for the origin of the tribe and the subtribes 4 5 Sonia Herrando-Morairaa,*, Juan Antonio Callejab, Mercè Galbany-Casalsb, Núria Garcia-Jacasa, Jian- 6 Quan Liuc, Javier López-Alvaradob, Jordi López-Pujola, Jennifer R. Mandeld, Noemí Montes-Morenoa, 7 Cristina Roquetb,e, Llorenç Sáezb, Alexander Sennikovf, Alfonso Susannaa, Roser Vilatersanaa 8 9 a Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s.n., 08038 Barcelona, Spain 10 b Systematics and Evolution of Vascular Plants (UAB) – Associated Unit to CSIC, Departament de 11 Biologia Animal, Biologia Vegetal i Ecologia, Facultat de Biociències, Universitat Autònoma de 12 Barcelona, ES-08193 Bellaterra, Spain 13 c Key Laboratory for Bio-Resources and Eco-Environment, College of Life Sciences, Sichuan University, 14 Chengdu, China 15 d Department of Biological Sciences, University of Memphis, Memphis, TN 38152, USA 16 e Univ. Grenoble Alpes, Univ. Savoie Mont Blanc, CNRS, LECA (Laboratoire d’Ecologie Alpine), FR- 17 38000 Grenoble, France 18 f Botanical Museum, Finnish Museum of Natural History, PO Box 7, FI-00014 University of Helsinki, 19 Finland; and Herbarium, Komarov Botanical Institute of Russian Academy of Sciences, Prof. Popov str. 20 2, 197376 St. Petersburg, Russia 21 22 *Corresponding author at: Botanic Institute of Barcelona (IBB, CSIC-ICUB), Pg. del Migdia, s. n., ES- 23 08038 Barcelona, Spain. E-mail address: [email protected] (S. Herrando-Moraira). 24 25 Abstract 26 Classification of the tribe Cardueae in natural subtribes has always been a challenge due to the lack of 27 support of some critical branches in previous phylogenies based on traditional Sanger markers. -

Medical Ethnobotany of the Albanian Alps in Kosovo
Medical ethnobotany of the Albanian Alps in Kosovo Behxhet Mustafa, University of Prishtina Avni Hajdari, University of Prishtina Feriz Krasniqi, Kosovo Academy of Sciences and Arts Esat Hoxha, University of Prishtina Hatixhe Ademi, University of Prishtina Cassandra Quave, Emory University Andrea Pieroni, Univ Gastron Sci Journal Title: Journal of Ethnobiology and Ethnomedicine Volume: Volume 8 Publisher: BioMed Central | 2012-01-28, Pages 6-6 Type of Work: Article | Final Publisher PDF Publisher DOI: 10.1186/1746-4269-8-6 Permanent URL: https://pid.emory.edu/ark:/25593/rnh2w Final published version: http://dx.doi.org/10.1186/1746-4269-8-6 Copyright information: © 2012 Mustafa et al; licensee BioMed Central Ltd. Accessed September 26, 2021 3:06 AM EDT Mustafa et al. Journal of Ethnobiology and Ethnomedicine 2012, 8:6 http://www.ethnobiomed.com/content/8/1/6 JOURNAL OF ETHNOBIOLOGY AND ETHNOMEDICINE RESEARCH Open Access Medical ethnobotany of the Albanian Alps in Kosovo Behxhet Mustafa1, Avni Hajdari1*, Feriz Krasniqi2, Esat Hoxha1, Hatixhe Ademi1, Cassandra L Quave3 and Andrea Pieroni4 Abstract Background: Ethnobotanical studies are crucial in South-Eastern Europe for fostering local development and also for investigating the dynamics of Traditional Environmental Knowledge (TEK) related to plants in one of the most crucial European hotspots for biocultural diversity. The current medico-ethnobotanical survey was conducted in rural alpine communities in Kosovo. The aims of the study were twofold: 1) to document the state of TEK of medicinal plants in these communities; 2) to compare these findings with that of similar field studies previously conducted among local populations inhabiting the Montenegrin and Albanian side of the same Alpine range. -
Field Grown Cut Flowers
Nursery FACTSHEET September 2015 Field Grown Cut Flowers INTRODUCTION The culture of field grown flowers is an area of floriculture that is generating a lot of interest and is enjoying a steady growth rate. It provides a way to enter the floriculture industry without the $100 to $150 per square metre capital costs that are involved in some greenhouse crops. Recently, the largest area of growth has been in the specialty cut flowers as opposed to the more traditional field grown crops like statice, dahlias and gypsophila. As gardening increases in popularity, home consumers are becoming familiar with the many new and different flower species. In turn, consumers are starting to look for and demand these flowers in floral design work. Site Selection Whether you plan to lease or own the land, there are basic, yet important, site considerations (see Table 1). It is easier if you start with a suitable site rather than try to modify it later. Table 1. Considerations when selecting a production site Soil: It should be fertile and well drained. Soil tests are a basic management tool. Even if you are familiar with the soil in the area, it must be tested to determine pH, organic matter and nutrient levels. A pH of 6.0–6.5 is suitable for most cuts. Know the requirements of your crop before you make any major changes. Water: Good quality water must be available in sufficient quantities. Have the water source tested to determine essentials like pH and EC (salinity). Terrain: Flat land is easier to work. Watch out for low lying pockets that might be prone to early and late frosts, or flooding during the wet months. -

Phylogenetic Uncertainty and Fossil Calibration of Asteraceae Chronograms LETTER Jose L
LETTER Phylogenetic uncertainty and fossil calibration of Asteraceae chronograms LETTER Jose L. Panero1 Barreda et al. (1) claim a Cretaceous fossil pollen type is an opposed to other Barnadesioideae. Encoding “columel- extinct Asteraceae. Concluding this pollen type is “nested late layer visibility under light microscopy” (character 19) within Dasyphyllum (crown representative),” they calibrate results in unintentional character weighting. Exine thick- a Dasyphyllum + Barnadesia crown node (Dasyphyllum ness (character 22) is much smaller in Dasyphyllum crown absent) and estimate an 85.9-Ma Asteraceae crown inerme and Dasyphyllum velutinum than in other Dasy- age that potentially compresses asterid evolution by tens phyllum spp. (3) that would be scored as other Barnade- of millions of years. However, the bootstrap majority con- sioideae and different from the fossil had they been sensus topology reported could not be reproduced from sampled. Characters 19, 21, and 22 clearly contribute the data; instead, the fossil resolved in a trichotomy with to place the fossil with Dasyphyllum. Character 17 as- Calyceraceae and Asteraceae. Thus, unambiguous assign- sumes that concavities distributed asymmetrically ment of these pollen grains to Asteraceae is premature. (sometimes absent) along the intercolpal region in the Paleocene, not Cretaceous, mean ages of Asteraceae fossil are homologous with symmetrically distributed result from calibration placement consistent with the intercolpal concavities in extant taxa and not the result fossil’s phylogenetic position in the reproduced bootstrap of compression forces during fossilization (figure 4 in tree. Calibration at the Asteraceae + Calyceraceae crown ref. 1). This character is scored as “present” in the fossil node (second calibration scenario; figure S5A and table (table S1 in ref.