Development of Enzyme-Linked Immunosorbent Assays for the Detection of Mutagenic Metabolites of the Herbicide Alachlor
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
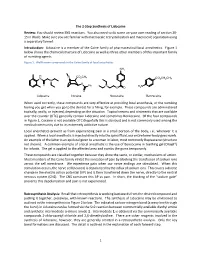
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

1. A. the First Reaction Is a Friedel-Crafts Acylation (FCA), Where the Major Product Is the Para- Isomer (60% Isolated Yield)
1. a. The first reaction is a Friedel-Crafts Acylation (FCA), where the major product is the para- isomer (60% isolated yield). The second reaction is a nitration, where the incoming electrophile (nitronium ion) is directed to the ortho position of the methoxy group. The last reaction is a Wolff-Kishner reduction that converts the acetyl group into an ethyl group. The nitro group does not react under these conditions. OCH OCH OCH OCH3 3 3 3 NO2 NO2 N2H4/KOH CH3COCl/AlCl3 H2SO4/HNO3 0 oC O CH 3 O CH3 CH3 (A) Reaction 1 (B) Reaction 2 (C) Reaction 3 (P) b. The best solvent for the FC-acylation is dichloromethane. Tetrahydrofuran is a fairly strong Lewis base, which would react and deactivate the AlCl3 catalyst. Ethanol would also react with AlCl3 and form alcoholates, which are inactive at FCA catalyst. Dichloromethane is polar enough to dissolve all three compounds but does not form adducts with AlCl3. Thus, aluminum chloride maintains its Lewis acidity. 3+ c. As discussed in lecture, AlCl3*6 H2O is not suitable as catalyst because the Al is not a strong Lewis acid anymore. In addition, larger amounts of water would destroy the acetyl chloride as well (=hydrolysis, CH3COCl + H2O ---- > CH3COOH + HCl). Consequently, the reaction would not proceed in the desired fashion. 3+ OH2 H2O OH2 Al H2O OH2 OH2 d. In order to determine the yield, one has to calculate the number of moles of the reactant and the product. nA = 1.90 mL * 0.996 g/mL/108.14 g/mol = 17.5 mmol nCH3COCl = 2.49 mL * 1.104 g/mL/78.5 g/mol = 35.0 mmol nAlCl3 = 4.67 g/133.5 g/mol = 35.0 mmol Compound (A) is the limiting reagent. -

United States Patent (19) (11) 4,129,595 Suzuki 45) Dec
United States Patent (19) (11) 4,129,595 Suzuki 45) Dec. 12, 1978 (54) PREPARATION OF CHLOROACETYL (56) References Cited CHLORDE PUBLICATIONS E. E. Blaise et al., Comptes Rendus (France), vol. 174, 75 Inventor: Shigeto Suzuki, San Francisco, Calif. pp. 1173-1174, (1922), (Chem. Abstr., vol. 16, 2480). 73) Assignee: Chevron Research Company, San Primary Examiner-Gerald A. Schwartz Francisco, Calif. Attorney, Agent, or Firm-D. A. Newell; John Stoner, Jr. (21) Appl. No.: 891,429 57 ABSTRACT Chloroacetyl chloride is prepared by reacting glycolic (22) Filed: Mar. 29, 1978 acid with thionyl chloride in the presence of nitrogen 51) int. C.’.............................................. CO7C 51/58 containing organic compound orphosphine compound. 52 U.S. C. ................................................ 260/544 Y 58 Field of Search .................................... 260/544 Y 3 Claims, No Drawings 4,129,595 1. 2 glycolic acid with thionyl chloride in the presence of a PREPARATION OF CHLOROACETYL CHLORDE catalytic amount of nitrogen-containing hydrocarbyl organic compound or hydrocarbyl phosphine com BACKGROUND OF THE INVENTION pound at high conversion and yield in accordance with 1. Field of the Invention 5 the present invention. The present invention relates to the preparation of chloroacetyl chloride. More particularly, the invention DETALED DESCRIPTION OF THE relates to the preparation of chloroacetyl chloride by INVENTION reacting glycolic acid with thionyl chloride in the pres The nitrogen-containing hydrocarbyl organic com ence of nitrogen-containing -

Ketene Reactions. I. the Addition of Acid Chlorides
KETENE REACTIONS. I. THE ADDITION OF ACID CHLORIDES TO DIMETHYLKETENE. II. THE CYCLOADDITION OF KETENES TO CARBONYL COMPOUNDS APPROVED: Graduate Committee: Major Professor Committee Member.rr^- Committee Member Committee Member Director of the Department of Chemistry Dean' of the Graduate School Smith, Larry, Ketene Reactions. I. The Addition of Acid Chlorides to DimethyIketene. II. The Cycloaddition of Ketenes to Carbonvl Compounds. Doctor of Philosophy (Chemistry), December, 1970, 63 pp., 3 tables, bibliography, 62 titles. Part I describes the addition of several acid chlorides to dimethylketene. The resulting 3-ketoacid chlorides were isolated and characterized. The reactivities of acid chlorides were found to parallel the parent acid pKa's. A reactivity order of ketenes toward acid chlorides was established. Dimethylketene is more reactive than ketene which is more reactive than diphenylketene. Attempts to effect the addition of an acid halide to a ketene produced by in situ dehydro- halogenation yielded a-halovinyl esters. The addition of acid chlorides to ketenes was concluded to be an ionic process dependent upon the nucleophilic character of the ketene oc- carbon and the polarity of the carbon-chlorine bond in the acid chloride. Part II describes the cycloaddition of several aldo- ketenes to chloral. The ketenes were generated in situ by dehydrohalogenation and dehalogenation of appropriately substituted acyl halides. Both cis- and trans-4-trichloro- Miyl-2-oxetanones are produced in the cycloadditions with the sterically hindered cis isomer predominating. Isomer distributions were determined by vpc or nmr analysis of the reaction solutions. Production of the ketenes by dehalo- genation resulted in enhanced reactivity of the carbonyl compounds. -

Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid
Asian Journal of Chemistry; Vol. 26, No. 2 (2014), 475-480 http://dx.doi.org/10.14233/ajchem.2014.15484 Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid * JIAN-WEI XUE , JIAN-PENG ZHANG, BO WU, FU-XIANG LI and ZHI-PING LV Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, P.R. China *Corresponding author: Fax: +86 351 6111178; Tel: +86 351 60105503; E-mail: [email protected] Received: 14 March 2013; Accepted: 17 May 2013; Published online: 15 January 2014; AJC-14570 The process of acetic acid catalysis and chlorination for synthesizing chloroacetic acid can exist in not only gas phase but also liquid phase. In this paper, the gas-phase reaction mechanism of the synthesis of chloroacetic acid was studied. Due to the high concentration of acetic acid and the better reaction mass transfer in the liquid-phase reaction, the generation amount of the dichloroacetic acid was higher than that in the gas-phase reaction. Under the solution distillation, the concentration of acetyl chloride, whose boiling point is very low, was very high in the gas phase, sometimes even up to 99 %, which would cause the acetyl chloride to escape rapidly with the hydrogen chloride exhaust, so that the reaction slowed down. Therefore, series reactions occured easily in the gas-phase reaction causing the amount of the dichloroacetic acid to increase. Keywords: Gas phase, Catalysis, Chlorination, Chloroacetic acid, Acetic acid. INTRODUCTION Martikainen et al.3 summed up the reaction mechanism that was consistent with a mechanism found by Sioli according Chloroacetic acid is not only a fine chemical product but to the system condition experiment and systematic theoretical also an important intermediate in organic synthesis. -

Methods for the Acylation of Aromatic Amino Com- Pounds and Ureas, with Especial Refer- Ence to Chloroacetylation
ACYLATION OF AROMATIC AMINO COMPOUNDS AND UREAS. 1439 m-Aminobenzoy1urea.-The necessary m-nitrobenzoylurea was pre- pared by boiling the acid chloride and urea in benzene for IZ hours in- stead of following Griess' method' of fusing the components at 150'. The amino urea was obtained in the same way as the o-isomer, except that it was necessary to evaporate the alcohol before the substance sepa- rated completely on cooling. The yield was 6 g. from IO g. of the nitro compound. As Griess' description of m-aminobenzoylurea is not com- plete, the following is appended: When rapidly heated it melts with gas evolution at about ZIO', resolidifying and then remelting at about 275-80'. It is readily diazotized, in contradistinction to the o-isomer, yielding a scarlet color with R-salt, and dissolves readily in boiling water or 95% alcohol. Kjeldahl: 0.0997 g. subst.; 16.50 cc. 0.1 N HC1. Calcd. for CsHpOlNa: N, 23.46%. Found: N, 23.18%. Nsw YORKCITY. [CONTRIBUTION FROM THE LABORATORIESOF THE ROCKEFELLERINSTITUTE FOR MEDICALRESEARCH. ] METHODS FOR THE ACYLATION OF AROMATIC AMINO COM- POUNDS AND UREAS, WITH ESPECIAL REFER- ENCE TO CHLOROACETYLATION. BY WALTERA. JACOBS AND MICHAELHBIDBLBERGER. Received May 5, 1917. The importance of the halogenacetyl compounds has been demonstrated by their frequent use in organic synthesis. The great reactivity of the halogen atom has permitted their use in practically all the reactions in which alkyl halides have been employed. In the work of the authors on the quaternary salts of hexamethylenetetramine2 one phase of their usefulness in synthetic work in chemotherapy was demonstrated. -

Chloroacetyl Chloride
Chloroacetyl chloride sc-239516 Material Safety Data Sheet Hazard Alert Code Key: EXTREME HIGH MODERATE LOW Section 1 - CHEMICAL PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME Chloroacetyl chloride STATEMENT OF HAZARDOUS NATURE CONSIDERED A HAZARDOUS SUBSTANCE ACCORDING TO OSHA 29 CFR 1910.1200. NFPA FLAMMABILITY0 HEALTH3 HAZARD INSTABILITY1 SUPPLIER Company: Santa Cruz Biotechnology, Inc. Address: 2145 Delaware Ave Santa Cruz, CA 95060 Telephone: 800.457.3801 or 831.457.3800 Emergency Tel: CHEMWATCH: From within the US and Canada: 877-715-9305 Emergency Tel: From outside the US and Canada: +800 2436 2255 (1-800-CHEMCALL) or call +613 9573 3112 PRODUCT USE Preparation of chloroacetophenone; intermediate; tear gas. SYNONYMS C2-H2-Cl2-O, ClCH2COCl, "acetyl chloride, chloro-", "chloroacetic acid chloride", "chloroacetic chloride", "monochloroacetyl chloride" Section 2 - HAZARDS IDENTIFICATION CANADIAN WHMIS SYMBOLS EMERGENCY OVERVIEW RISK Reacts violently with water. Causes severe burns. Risk of serious damage to eyes. Toxic: danger of serious damage to health by prolonged exposure through inhalation. Toxic by inhalation, in contact with skin and if swallowed. 1 of 20 Very toxic to aquatic organisms. POTENTIAL HEALTH EFFECTS ACUTE HEALTH EFFECTS SWALLOWED • The material can produce severe chemical burns within the oral cavity and gastrointestinal tract following ingestion. • Toxic effects may result from the accidental ingestion of the material; animal experiments indicate that ingestion of less than 40 gram may be fatal or may produce serious damage to the health of the individual. • Ingestion of acidic corrosives may produce burns around and in the mouth. the throat and esophagus. Immediate pain and difficulties in swallowing and speaking may also be evident. -

United States Patent (19) 11 3,956,304 Schwarze Et Al
United States Patent (19) 11 3,956,304 Schwarze et al. (45 May 11, 1976 54 PREPARATION OF 56 References Cited 2-METHYL-4-ISOPROPYLDENE-2 UNITED STATES PATENTS OXAZOLN-5-ONE 2,569,80i 10/1951 Cook et al.......................... 260/307 75 Inventors: Werner Schwarze, Frankfurt; Gerd OTHER PUBLICATIONS Schreyer, Grossauheim both of Elderfield- “Heterocyclic Compounds'-Vol. 5-John Germany Wiley Press (1957) - pp. 338-339. 73 Assignee: Deutsche Gold- und Silber-Scheideanstalt vormals Primary Examiner-Raymond V. Rush Roessler, Germany Attorney, Agent, or Firm-Cushman, Darby & 22 Filed: Sept. 12, 1974 Cushman 21 Appl. No.: 505,607 57) ABSTRACT N-acetyl-DL-penicillamine is prepared from DL-valine 30 Foreign Application Priority Data by converting the DL-valine into 2-methyl-4- Sept. 2, 1973 Germany............................ 2345.835 isopropylidene-2-oxazolin-5-one using chloroacetyl chloride at 50 to 150°C. and then converting the 2 52 U.S. Cl.............................................. 260/307 A methyl-4-isopropylidene-2-oxazolin-5-one into the 5 Int. Cl.’........................................ C07D 263/42 N-acetyl-DL-penicillamine. 58 Field of Search................................. 260/307 A 13 Claims, No Drawings 3,956,304 1 2 ceed a molar ratio of chloroacetyl chloride to valine of PREPARATION OF 4 to 1. 2-METHYL-4-ISOPROPYLIDENE-2-OXAZOLIN The reaction takes place in a suitable manner at S-ONE temperatures between about 50' and 150°C., prefer ably between 70 and 120°C. The pressure can range The invention is concerned with a process for the widely and is not critical. However, it is recommended production of N-acetyl-DL-penicillamine from DL in order to use simple apparatus to employ normal valine by converting the valine into 2-methyl-4-iso pressure, or if necessary moderately reduced or ele propylidene-2-oxazolin-5-one using chloroacetyl chlor vated pressures. -

UC Santa Barbara UC Santa Barbara Electronic Theses and Dissertations
UC Santa Barbara UC Santa Barbara Electronic Theses and Dissertations Title Machine Learning for Addressing Data Deficiencies in Life Cycle Assessment Permalink https://escholarship.org/uc/item/2vc7t19w Author Song, Runsheng Publication Date 2019 Peer reviewed|Thesis/dissertation eScholarship.org Powered by the California Digital Library University of California UNIVERSITY OF CALIFORNIA Santa Barbara Machine Learning for Addressing Data Deficiencies in Life Cycle Assessment A dissertation submitted in partial satisfaction of the requirements for the degree Doctor of Philosophy in Environmental Science and Management by Runsheng Song Committee in charge: Professor Sangwon Suh, Co-Chair Professor Arturo A. Keller, Co-Chair Professor Krzysztof Janowicz March 2019 The dissertation of Runsheng Song is approved. _____________________________________________ Arturo A. Keller _____________________________________________ Krzysztof Janowicz _____________________________________________ Sangwon Suh, Committee Chair March 2019 Machine Learning for Addressing Data Deficiencies in Life Cycle Assessment Copyright © 2019 by Runsheng Song iii ACKNOWLEDGEMENTS I have the privilege to work with a group of smart people during my stay at the Bren School of Environmental Science and Management, UC Santa Barbara. I would like to first give my thanks to Prof. Sangwon Suh, my PhD advisor, who provides me countless guide, and offered invaluable helps during many difficult times over my PhD career. I would like to thank Prof. Arturo A. Keller, who is always encouraging and generous when I was confused or making mistake over the development of my research. I also want to thank Prof. Krzysztof Janowicz, who shines his wisdom from another field to my PhD research, and always pointing out the room for improvement in my study which I could never figure out without him. -

Department of Labor
Vol. 79 Friday, No. 197 October 10, 2014 Part II Department of Labor Occupational Safety and Health Administration 29 CFR Parts 1910, 1915, 1917, et al. Chemical Management and Permissible Exposure Limits (PELs); Proposed Rule VerDate Sep<11>2014 17:41 Oct 09, 2014 Jkt 235001 PO 00000 Frm 00001 Fmt 4717 Sfmt 4717 E:\FR\FM\10OCP2.SGM 10OCP2 mstockstill on DSK4VPTVN1PROD with PROPOSALS2 61384 Federal Register / Vol. 79, No. 197 / Friday, October 10, 2014 / Proposed Rules DEPARTMENT OF LABOR faxed to the OSHA Docket Office at Docket: To read or download (202) 693–1648. submissions or other material in the Occupational Safety and Health Mail, hand delivery, express mail, or docket go to: www.regulations.gov or the Administration messenger or courier service: Copies OSHA Docket Office at the address must be submitted in triplicate (3) to the above. All documents in the docket are 29 CFR Parts 1910, 1915, 1917, 1918, OSHA Docket Office, Docket No. listed in the index; however, some and 1926 OSHA–2012–0023, U.S. Department of information (e.g. copyrighted materials) Labor, Room N–2625, 200 Constitution is not publicly available to read or [Docket No. OSHA 2012–0023] Avenue NW., Washington, DC 20210. download through the Web site. All submissions, including copyrighted RIN 1218–AC74 Deliveries (hand, express mail, messenger, and courier service) are material, are available for inspection Chemical Management and accepted during the Department of and copying at the OSHA Docket Office. Permissible Exposure Limits (PELs) Labor and Docket Office’s normal FOR FURTHER INFORMATION CONTACT: business hours, 8:15 a.m.