Ketene Reactions. I. the Addition of Acid Chlorides
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

UNITED STATES PATENT of FICE 1926,642 PROCESS of OBTAINING REACTION PRODUCTS of RETENE Charles O
Patented Sept. 12, 1933 1926,642 UNITED STATES PATENT of FICE 1926,642 PROCESS OF OBTAINING REACTION PRODUCTS OF RETENE Charles O. Young and George H. Reid, South Charleston, W. Wa, assignors to Carbide & Carbon Chemicals. Corporation, a corporation of New York No Drawing. Application December 2, 1930 Serial No. 502,005 8 Claims. (C. 260-06) The invention is an improved process of mak plied in any desired manner, for example the ap ing reaction products of ketene (CH2=C-O). In paratus for quenching the hot vapors may take general, the process of our invention comprises the form of a packed tower scrubber, spray-scrub thermally decomposing a substance which will ber or any other suitable means for rapidly cool form ketene, and rapidly cooling the hot gaseous ing the hot vapors in intimate contact with the 80 products of this pyrolysis in intimate contact with abSOrbing medium. a reacting absorbing medium. The absorption or quenching means may be The principal object of the invention is to pro operated at ordinary temperatures, and in such Wide a method of making ketene and reaction a case the resultant liquid will contain the reac 0 products thereof which will result in the forma tion product of ketene and the absorbing medium, 65 tion of a maximum amount of valuable product excess absorbing medium and condensed un and which will minimize losses of the ketene changed acetone. The liquid may be circulated formed. until Sufficiently reacted with ketene and then sep In the manufacture of ketene by the pyrolysis arated from the diluent acetone, or it may be oth 15 of organic compounds, e.g., acetone, two primary erwise treated for the separation or recovery of O difficulties in Securing useful quantities of ketene its several constituents. -

US EPA Inert (Other) Pesticide Ingredients
U.S. Environmental Protection Agency Office of Pesticide Programs List of Inert Pesticide Ingredients List 3 - Inerts of unknown toxicity - By Chemical Name UpdatedAugust 2004 Inert Ingredients Ordered Alphabetically by Chemical Name - List 3 Updated August 2004 CAS PREFIX NAME List No. 6798-76-1 Abietic acid, zinc salt 3 14351-66-7 Abietic acids, sodium salts 3 123-86-4 Acetic acid, butyl ester 3 108419-35-8 Acetic acid, C11-14 branched, alkyl ester 3 90438-79-2 Acetic acid, C6-8-branched alkyl esters 3 108419-32-5 Acetic acid, C7-9 branched, alkyl ester C8-rich 3 2016-56-0 Acetic acid, dodecylamine salt 3 110-19-0 Acetic acid, isobutyl ester 3 141-97-9 Acetoacetic acid, ethyl ester 3 93-08-3 2'- Acetonaphthone 3 67-64-1 Acetone 3 828-00-2 6- Acetoxy-2,4-dimethyl-m-dioxane 3 32388-55-9 Acetyl cedrene 3 1506-02-1 6- Acetyl-1,1,2,4,4,7-hexamethyl tetralin 3 21145-77-7 Acetyl-1,1,3,4,4,6-hexamethyltetralin 3 61788-48-5 Acetylated lanolin 3 74-86-2 Acetylene 3 141754-64-5 Acrylic acid, isopropanol telomer, ammonium salt 3 25136-75-8 Acrylic acid, polymer with acrylamide and diallyldimethylam 3 25084-90-6 Acrylic acid, t-butyl ester, polymer with ethylene 3 25036-25-3 Acrylonitrile-methyl methacrylate-vinylidene chloride copoly 3 1406-16-2 Activated ergosterol 3 124-04-9 Adipic acid 3 9010-89-3 Adipic acid, polymer with diethylene glycol 3 9002-18-0 Agar 3 61791-56-8 beta- Alanine, N-(2-carboxyethyl)-, N-tallow alkyl derivs., disodium3 14960-06-6 beta- Alanine, N-(2-carboxyethyl)-N-dodecyl-, monosodium salt 3 Alanine, N-coco alkyl derivs. -

Nov 15, Ketene Chemistry and the Application in Synthesis by Xuan Zhou
Ketene chemistry and the application in synthesis Dong group at UT Austin Xuan Zhou Nov 14, 2013 Ketene chemistry Content • A brief history of ketene • Type of ketenes • Ketene preparation • Ketenes in synthesis Reviews about ketenes: T. T. Tidwell, Ketenes, 2nd ed., wiley interscience, Hoboken, NJ, 2006. T. T. Tidwell, Eur. J. Org. Chem. 2006, 563-576. T. T. Tidwell, Angew. Chem. Int. Ed. 2005, 44, 5778-5785. A brief history of ketene The first reported ketene: Diphenylketene Wedekind Ketene and its Dimer: N.T.M. Wilsmore, J. Chem. Soc. 1907, 91, 1938 Asymmetric reactions of ketene: H. Pracejus, Justus liebigs Ann. Chem. 1969, 722, 1-11 Bisketenes First prepared bisketenes by Wolf in 1906 O. Diels, B. Wolf, Ber. Dtsch. Chem. Ges. 1906, 39, 689-697 First observed bisketenes in 1982 G. Maier, H. P. Reisenauer, T. Sayrac, Chem. Ber. 1982, 115, 2192 Substituent effects of ketene Melvin Newman Shchukovskaya Cycloadditions of ketenes Lee Irvin Smith Derek H.R. Barton Angew. Chem. Int. Ed. 2005, 44, 5779-5785 Type of ketenes Carbon-substituted ketenes • Alkylketenes 2 3 4 • Alkenylketenes 5 6 7 • alkynylketenes and cyanoketenes 8 9 10 Type of ketenes • Arylketenes 1 2 3 • Acylketenes 4 5 6 • Imidoylketenes 7 azetinones 8 Nitrogen-substituted ketenes 1 Nitroketene Azidoketene Isocyanatoketene 2 Oxygen-substituted ketenes 3 4 5 6 Halogen-substituted ketenes 1 2 3 4 Silyl-ketenes 5 6 7 Phosphorous, sulfur, metal-substituted and bis ketenes 1 2 5 6 7 8 9 10 11 12 Ketene preparation Ketenes from ketene dimers •Pyrolysis of ketene dimer 1 2 3 4 •Photolysis -

The Interactions and Reactions of Atoms and Molecules on the Surfaces of Model Interstellar Dust Grains
The Interactions and Reactions of Atoms and Molecules on the Surfaces of Model Interstellar Dust Grains A thesis submitted for the degree of Doctor of Philosophy Helen Jessica Kimber Department of Chemistry University College London 2016 -I, Helen Jessica Kimber, confirm that the work presented in this thesis is my own. Where information has been derived from other sources, I confirm that this has been indicated in the thesis. Signed, i Abstract The elemental composition of the known universe comprises almost exclusively light atoms (~99.9% hydrogen and helium). However, to date, close to 200 different molecules have been detected in the interstellar medium (ISM) where their distribution is far from uniform. The vast majority of these molecules are contained within vast clouds of gas and dust referred to as interstellar clouds. Within these interstellar clouds, many of the molecules present are formed via gas-phase ion-neutral reactions. However, there are several molecules for which known gas-phase kinetics cannot account for observed gas-phase abundances. As a result, reactions occurring on the surface of interstellar dust grains are invoked to account for the observed abundances of some of these molecules. This thesis presents results of experimental investigations into the interaction and reactions of atoms and molecules on the surface of model interstellar dust grains. Chapters three and four present results for the reaction of (3P)O on molecular ices. Specifically, the reaction of (3P)O and propyne or acrylonitrile. After a one hour dosing period, temperature programmed desorption (TPD), coupled with time-of-flight mass spectrometry (TOFMS), are used to identify (3P)O addition products. -
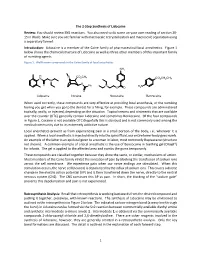
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Lonza's Chemical Network Diketene and HCN Derivatives, Heterocycles
Lonza’s chemical network Diketene and HCN derivatives, heterocycles and basic chemicals catalog Pharma&Biotech Nutrition Agriculture MaterialsScience PersonalCare Diketene and HCN derivatives, heterocycles and basic chemicals catalog Content About Lonza 3 Introduction 4 Diketene / ketene derivatives 6 Esters 6 Arylides 7 Alkylamides 8 Pyrazolones 9 Dehydroacetic acid 9 Lonzamon monomers 10 Other diketene derivatives 10 HCN derivatives 12 Heterocycles 14 Basic chemicals 16 Others 17 Alphabetical index 18 About Lonza Lonza is the global leader in the production and support of active phar- From 1897 to the present day combining Swiss tradition with global maceutical ingredients both chemically and biotechnologically. Biophar- experience, the company has had an enterprising character, adapting maceuticals are one of the key growth drivers of the pharmaceutical and its offerings and services to the needs of customers and to changing biotechnology industries. technologies. Throughout our history, we have maintained a strong culture of performance, results and dependability that is valued by all Lonza has strong capabilities in large and small molecules, peptides, of our customers. amino acids and niche bioproducts which play an important role in the development of novel medicines and healthcare products. In addition, Lonza’s cracker in Visp is the back bone of a comprehensive fully back- Lonza is a leader in cell-based research, endotoxin detection and cell ward integrated chemical network. Our product portfolio consists of therapy manufacturing. Furthermore, the company is a leading provider HCN- and diketene derivatives as well as basic chemicals which are key of chemical and biotech ingredients to the nutrition, hygiene, preserva- raw materials and intermediates for many sophisticated applications. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

Photochemical Generation of Carbenes and Ketenes from Phenanthrene-Based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene
Colby College Digital Commons @ Colby Honors Theses Student Research 2017 Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene Tarini S. Hardikar Student Tarini Hardikar Colby College Follow this and additional works at: https://digitalcommons.colby.edu/honorstheses Part of the Organic Chemistry Commons Colby College theses are protected by copyright. They may be viewed or downloaded from this site for the purposes of research and scholarship. Reproduction or distribution for commercial purposes is prohibited without written permission of the author. Recommended Citation Hardikar, Tarini S. and Hardikar, Tarini, "Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene" (2017). Honors Theses. Paper 948. https://digitalcommons.colby.edu/honorstheses/948 This Honors Thesis (Open Access) is brought to you for free and open access by the Student Research at Digital Commons @ Colby. It has been accepted for inclusion in Honors Theses by an authorized administrator of Digital Commons @ Colby. Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene TARINI HARDIKAR A Thesis Presented to the Department of Chemistry, Colby College, Waterville, ME In Partial Fulfillment of the Requirements for Graduation With Honors in Chemistry SUBMITTED MAY 2017 Photochemical Generation of Carbenes and Ketenes from Phenanthrene-based Precursors Part I: Dimethylalkylidene Part II: Diphenylketene TARINI HARDIKAR Approved: (Mentor: Dasan M. Thamattoor, Professor of Chemistry) (Reader: Rebecca R. Conry, Associate Professor of Chemistry) “NOW WE KNOW” - Dasan M. Thamattoor Vitae Tarini Shekhar Hardikar was born in Vadodara, Gujarat, India in 1996. She graduated from the S.N. -

The Development of the First Catalyzed Reaction of Ketenes and Imines: Catalytic, Asymmetric Synthesis of Â-Lactams Andrew E
Published on Web 00/00/0000 The Development of the First Catalyzed Reaction of Ketenes and Imines: Catalytic, Asymmetric Synthesis of â-Lactams Andrew E. Taggi, Ahmed M. Hafez, Harald Wack, Brandon Young, Dana Ferraris, and Thomas Lectka* Contribution from the Department of Chemistry, Johns Hopkins UniVersity, 3400 North Charles Street, Baltimore, Maryland 21218 Received February 5, 2002 Abstract: We report practical methodology for the catalytic, asymmetric synthesis of â-lactams resulting from the development of a catalyzed reaction of ketenes (or their derived zwitterionic enolates) and imines. The products of these asymmetric reactions can serve as precursors to a number of enzyme inhibitors and drug candidates as well as valuable synthetic intermediates. We present a detailed study of the mechanism of the â-lactam forming reaction with proton sponge as the stoichiometric base, including kinetics and isotopic labeling studies. Stereochemical models based on molecular mechanics (MM) calculations are also presented to account for the observed stereoregular sense of induction in our reactions and to provide a guidepost for the design of other catalyst systems. Introduction this structural motif a worthwhile goal for the synthetic organic 10 The clinical relevance of â-lactams continues to expand at a chemist, thus the synthesis of these nonantibiotic â-lactams surprising rate. Although their use as antibiotics is being will be the focus of this contribution. While considerable effort compromised to some extent by bacterial resistance pressures,1 has been put into synthetic methodology to construct the basic recently â-lactams (especially nonnatural ones) have achieved â-lactam skeleton, there have been few general methods many important nonantibiotic uses. -

US EPA, Inert (Other) Pesticide Ingredients in Pesticide Products
Inert Ingredients ordered by CAS Number Updated August 2004 CAS PREFIX NAME List No. 50-21-5 Lactic acid 4B 50-70-4 Sorbitol 4A 50-81-7 L- Ascorbic acid 4A 50-99-7 Dextrose 4A 51-03-6 Piperonyl butoxide 3 51-05-8 Procaine hydrochloride 3 51-55-8 Atropine 3 52-51-7 2- Bromo-2-nitro-propane-1,3-dio 3 54-21-7 Sodium salicylate 3 56-81-5 Glycerol (glycerin) 1,2,3 propanetriol 4A 56-86-0 L- Glutamic acid 3 56-95-1 Chlorhexidine diacetate 3 57-10-3 Hexadecanoic acid 4A 57-11-4 Stearic acid 4A 57-13-6 Urea 4A 57-48-7 D- Fructose 4B 57-50-1 Sugar 4A 57-55-6 Propylene glycol 4B 57-88-5 (3.beta.)- Cholest-5-en-3-ol 4B 58-08-2 1H- Purine-2,6-dione, 3,7-dihydro-1,3,7-trimethyl- 4B 58-56-0 Thiamine mononitrate 4B 58-85-5 Biotin 3 58-86-6 D- Xylose 4B 58-95-7 Vitamin E acetate 3 59-30-3 Folic acid 4B 59-40-5 N-(2- Quinoxalinyl)sulfanilide 3 59-67-6 Nicotinic acid 3 60-00-4 Ethylenediaminetetraacetic acid (EDTA) 4B 60-12-8 Benzeneethanol 3 60-29-7 Ethane, 1,1'-oxybis- 3 60-33-3 Linoleic acid 3 61-73-4 C.I. Basic Blue 9 3 62-33-9 Ethylenediaminetetraacetic acid (EDTA), calcium4B 62-54-4 Acetic acid, calcium salt 4A 63-42-3 D-(+)-Lactose 4A 63-68-3 L- Methionine 4B 64-02-8 Ethylenediaminetetraacetic acid (EDTA), tetraso4B 64-17-5 Ethanol 4B 64-18-6 Formic acid 3 64-19-7 Acetic acid 4B 64-86-8 Colchicine 3 65-85-0 Benzoic acid 4B 66-71-7 1,10- Phenanthroline 3 67-03-8 Thiamin hydrochloride 3 67-43-6 1,1,4,7,7- Diethylenetriaminepentaacetic acid 3 67-48-1 Choline chloride 4B 67-56-1 Methyl alcohol 3 67-63-0 2- Propanol 4B 67-64-1 Acetone 3 67-68-5 Dimethyl -

Acetic Anhydride
ACETIC ANHYDRIDE Method number: 102 Matrix: Air Target concentration: 5 ppm (20 mg/m3) OSHA PEL: 5 ppm (20 mg/m3) TWA ACGIH TLV: 5 ppm (20 mg/m3) ceiling Procedure: Samples are collected open face on glass fiber filters coated with veratrylamine and di-n-octyl phthalate. Samples are extracted with 50/50 (v/v) 2-propanol/toluene and analyzed by GC using a nitrogen- phosphorus detector (NPD). Recommended air volume and sampling rate: 7.5 L at 0.5 L/min ceiling 7.5 L at 0.05 L/min TWA Reliable quantitation limit: 0.094 ppm (0.39 mg/m3) Standard error of estimate at the target concentration: 6.4% Special caution: Ketene and acetyl chloride produce the same derivative as acetic anhydride. Coated filters should be used within a month of preparation. Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: October 1993 Chemist: Yihlin Chan Organic Methods Evaluation Branch OSHA Salt Lake Technical Center Salt Lake City, UT 84165-0200 1. General Discussion 1.1 Background 1.1.1 History In OSHA Method 82, acetic anhydride is collected on a glass fiber filter impregnated with 1-(2-pyridyl)piperazine, which reacts with the anhydride to form a derivative (Ref. 5.1). Attempts at using 1-(2-pyridyl)piperazine for the derivatization of maleic, phthalic, and trimellitic anhydrides failed, however, because the resulting derivatives of these anhydrides were found to be unstable. These anhydrides were derivatized with veratrylamine instead (Refs. 5.2-5.4).