Process for Preparing a Stabilized Chromium \III\ Propionate Solution
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acetic Anhydride
ACETIC ANHYDRIDE Method number: 102 Matrix: Air Target concentration: 5 ppm (20 mg/m3) OSHA PEL: 5 ppm (20 mg/m3) TWA ACGIH TLV: 5 ppm (20 mg/m3) ceiling Procedure: Samples are collected open face on glass fiber filters coated with veratrylamine and di-n-octyl phthalate. Samples are extracted with 50/50 (v/v) 2-propanol/toluene and analyzed by GC using a nitrogen- phosphorus detector (NPD). Recommended air volume and sampling rate: 7.5 L at 0.5 L/min ceiling 7.5 L at 0.05 L/min TWA Reliable quantitation limit: 0.094 ppm (0.39 mg/m3) Standard error of estimate at the target concentration: 6.4% Special caution: Ketene and acetyl chloride produce the same derivative as acetic anhydride. Coated filters should be used within a month of preparation. Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: October 1993 Chemist: Yihlin Chan Organic Methods Evaluation Branch OSHA Salt Lake Technical Center Salt Lake City, UT 84165-0200 1. General Discussion 1.1 Background 1.1.1 History In OSHA Method 82, acetic anhydride is collected on a glass fiber filter impregnated with 1-(2-pyridyl)piperazine, which reacts with the anhydride to form a derivative (Ref. 5.1). Attempts at using 1-(2-pyridyl)piperazine for the derivatization of maleic, phthalic, and trimellitic anhydrides failed, however, because the resulting derivatives of these anhydrides were found to be unstable. These anhydrides were derivatized with veratrylamine instead (Refs. 5.2-5.4). -

Title the Reaction of Methyl Chloride with Carbon Monoxide Author(S
Title The reaction of methyl chloride with carbon monoxide Author(s) Osugi, Jiro; Mizukami, Tetuo Citation The Review of Physical Chemistry of Japan (1964), 34(1): 7-18 Issue Date 1964-11-05 URL http://hdl.handle.net/2433/46843 Right Type Departmental Bulletin Paper Textversion publisher Kyoto University The Review of Physical Chemistry of Japan Vol. 34 No. 1 (1964) TftE REVIEW OF PHYSICAL CHE'HISTRY OF IAPA~, VDL. 34, No. 1, 1964 THE REACTION OF METHYL CHLORIDE WITH CARBON MONOXIDE BY ]IRD ~stlf,l ANDTETUO IVIIEUI:A]II* The reaction of methyl chloride witb carbon monoxide in the presence of pumice•anhydrous sodium borate catalyst yields mainly hydrogen chloride, methane, acetyl chloride and phosgene, and deposits carbon. Moreover, hydro• gen, methylene chloride, ethyl chloride and ethylene dichloride are obtained in a trace. From [he stand point of chemical kinetics the reaction is studied. From the initial rates of the products, the values of 13.1, I8.5, 23.2 and 27.Okcal/mole are obtained as the apparent activation energies {or the formation of acetyl chloride, phosgene, hydrogen chloride and methane respectively. Furthermore, the rates of formation of acetyl chloride and pbosgene are discussed. From the apparent rate constants, the apparent activation energies are obtained. The values are 19.0 kcal/male for acetyl chloride and 17.6 kcal/mole for phosgene. The reaction mechanism is discussed. Introduction Since a few years ago, carhop monoxide has been considered as one of the important materials for organic chemical industry, so there are some utilizations, such as s}•nthetic petroleum, Reppe's carhoxy- Iation reaction and oxo reaction etc. -

Prohibited and Restricted Chemical List
School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List PROHIBITED AND RESTRICTED CHEMICAL LIST Introduction After incidents of laboratory chemical contamination at several schools, DCPS, The American Association for the Advancement of Science (AAAS) and DC Fire and Emergency Management Services developed an aggressive program for chemical control to eliminate student and staff exposure to potential hazardous chemicals. Based upon this program, all principals are required to conduct a complete yearly inventory of all chemicals located at each school building to identify for the removal and disposal of any prohibited/banned chemicals. Prohibited chemicals are those that pose an inherent, immediate, and potentially life- threatening risk, injury, or impairment due to toxicity or other chemical properties to students, staff, or other occupants of the school. These chemicals are prohibited from use and/or storage at the school, and the school is prohibited from purchasing or accepting donations of such chemicals. Restricted chemicals are chemicals that are restricted by use and/or quantities. If restricted chemicals are present at the school, each storage location must be addressed in the school's written emergency plan. Also, plan maps must clearly denote the storage locations of these chemicals. Restricted chemicals—demonstration use only are a subclass in the Restricted chemicals list that are limited to instructor demonstration. Students may not participate in handling or preparation of restricted chemicals as part of a demonstration. If Restricted chemicals—demonstration use only are present at the school, each storage location must be addressed in the school's written emergency plan. Section 7: Appendices – October 2009 37 School Emergency Response Plan and Management Guide Prohibited and Restricted Chemical List Following is a table of chemicals that are Prohibited—banned, Restricted—academic curriculum use, and Restricted—demonstration use only. -

Ketene Reactions. I. the Addition of Acid Chlorides
KETENE REACTIONS. I. THE ADDITION OF ACID CHLORIDES TO DIMETHYLKETENE. II. THE CYCLOADDITION OF KETENES TO CARBONYL COMPOUNDS APPROVED: Graduate Committee: Major Professor Committee Member.rr^- Committee Member Committee Member Director of the Department of Chemistry Dean' of the Graduate School Smith, Larry, Ketene Reactions. I. The Addition of Acid Chlorides to DimethyIketene. II. The Cycloaddition of Ketenes to Carbonvl Compounds. Doctor of Philosophy (Chemistry), December, 1970, 63 pp., 3 tables, bibliography, 62 titles. Part I describes the addition of several acid chlorides to dimethylketene. The resulting 3-ketoacid chlorides were isolated and characterized. The reactivities of acid chlorides were found to parallel the parent acid pKa's. A reactivity order of ketenes toward acid chlorides was established. Dimethylketene is more reactive than ketene which is more reactive than diphenylketene. Attempts to effect the addition of an acid halide to a ketene produced by in situ dehydro- halogenation yielded a-halovinyl esters. The addition of acid chlorides to ketenes was concluded to be an ionic process dependent upon the nucleophilic character of the ketene oc- carbon and the polarity of the carbon-chlorine bond in the acid chloride. Part II describes the cycloaddition of several aldo- ketenes to chloral. The ketenes were generated in situ by dehydrohalogenation and dehalogenation of appropriately substituted acyl halides. Both cis- and trans-4-trichloro- Miyl-2-oxetanones are produced in the cycloadditions with the sterically hindered cis isomer predominating. Isomer distributions were determined by vpc or nmr analysis of the reaction solutions. Production of the ketenes by dehalo- genation resulted in enhanced reactivity of the carbonyl compounds. -

Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid
Asian Journal of Chemistry; Vol. 26, No. 2 (2014), 475-480 http://dx.doi.org/10.14233/ajchem.2014.15484 Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid * JIAN-WEI XUE , JIAN-PENG ZHANG, BO WU, FU-XIANG LI and ZHI-PING LV Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, P.R. China *Corresponding author: Fax: +86 351 6111178; Tel: +86 351 60105503; E-mail: [email protected] Received: 14 March 2013; Accepted: 17 May 2013; Published online: 15 January 2014; AJC-14570 The process of acetic acid catalysis and chlorination for synthesizing chloroacetic acid can exist in not only gas phase but also liquid phase. In this paper, the gas-phase reaction mechanism of the synthesis of chloroacetic acid was studied. Due to the high concentration of acetic acid and the better reaction mass transfer in the liquid-phase reaction, the generation amount of the dichloroacetic acid was higher than that in the gas-phase reaction. Under the solution distillation, the concentration of acetyl chloride, whose boiling point is very low, was very high in the gas phase, sometimes even up to 99 %, which would cause the acetyl chloride to escape rapidly with the hydrogen chloride exhaust, so that the reaction slowed down. Therefore, series reactions occured easily in the gas-phase reaction causing the amount of the dichloroacetic acid to increase. Keywords: Gas phase, Catalysis, Chlorination, Chloroacetic acid, Acetic acid. INTRODUCTION Martikainen et al.3 summed up the reaction mechanism that was consistent with a mechanism found by Sioli according Chloroacetic acid is not only a fine chemical product but to the system condition experiment and systematic theoretical also an important intermediate in organic synthesis. -

The Reaction of Acyl Halides with Aromatic Tertiary Amines
W. F. Day The R cacti on of AcljI Ha I ides w'tih mines THE REACTION OF ACYL HALIDES WITH AROMATIC TERTIARY AMINES BY WILBER FRANKLIN DAY THESIS FOR THE DEGREE OF BACHELOR OF SCIENCE IN CHEMISTRY COLLEGE OF LIBERAL ARTS AND SCIENCES UNIVERSITY OF ILLINOIS 1920 UNIVERSITY OF ILLINOIS ,.Juja.e....5 i$C... THIS IS TO CERTIFY THAT THE THESIS PREPARED UNDER MY SUPERVISION BY Wj, 1 b e r . F r ank1 i n . Da . y ENTITLED The...B^ag.t.l.Qn...ot... Ac.yl.. Hal.i.&e $....wlth Jjomati.c Teyt.i.ary. ne s . Am i IS APPROVED BY ME AS FULFILLING THIS PART OF THE REQUIREMENTS FOR THE Baglhel DEGREE OF... QX...Q&...8.Gte.TkGG , . I^T^t^r*^ . /^5^^T>?r^*^^. Instructor in Charge Approved :: aCjjC^.. HEAD OF DEPARTMENT OF Che.&i.3.t..ry. EA.BLE OF 90HS3SI-X8 page a JAuo ..u-~_;CL i.1 1 XBTBOEUCTIOM 2 HISTORICAL PART 5 THBOBETICAL PART 6 EXPERIMENTAL PART General 10 De termination of Velocity Constants 12 The Reaction Velocity of Benzoyl Chloride and Di butyl Aniline 12 The Reaction of Benzoyl Chloride with Di butyl Aniline in Xylene Solution 13 The Reaction Between Dimethyl Aniline and Benzene 3ulfonyl Chloride 13 The Reaction Between Di butyl Aniline and Benzene Sulfonyl Chloride 15 The Reaction Between Diethyl Aniline and Benzene oulfonyl Chloride 15 SUMMARY 16 BIBLIOGRAPHY 17 Digitized by the Internet Archive in 2013 http://archive.org/details/reactionofacylhaOOdayw -1- A C Eli OWLED GLffili T The author wishes to thank Doctor Oliver Earmn for the many kind and helpful suggestions oifered during the course of* this work. -
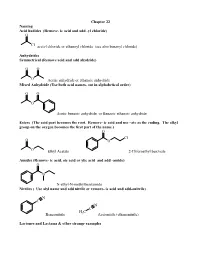
Remove- Ic Acid and Add -Yl Chloride) O
Chapter 22 Naming Acid hadides (Remove- ic acid and add -yl chloride) O Cl acetyl chloride or ethanoyl chloride (see also benzoyl chloride) Anhydrides Symmetrical (Remove acid and add ahydride) O O O Acetic anhydride or ethanoic anhydride Mixed Anhydride (Use both acid names, out in alphabetical order) O O O Acetic benzoic anhydride or Benzoic ethanoic anhydride Esters (The acid part becomes the root. Remove- ic acid and use –ate as the ending. The alkyl group on the oxygen becomes the first part of the name.) O Cl O O O Ethyl Acetate 2-Chloroethyl benzoate Amides (Remove- ic acid, oic acid or ylic acid and add -amide) O N N-ethyl-N-methylbenzamide Nitriles ( Use alyl name and add nitrile or remove- ic acid and add-onitrile) N C N C H C Benzonitrile 3 Acetonitile (ethanenitrile) Lactones and Lactams & other strange examples O O O NH 4-Butanolactone or γ-Butyrolactone 4-Butanolactam or γ-Butyrolactam O OEt EtO O Diethylsuccinate O O O NH O sucinnic anhydride O succinamide Reactions of acids halides. Preparation O O SOCl2 OH Cl Sample Mechanism: Acetyl chloride plus dimethyl amine. O O 2 eq. (CH3)2NH CH N 3 Cl H C 3 CH N 3 H C 3 H O : O : Cl Cl H C H C + 3 3 N N H3C H CH3 .. CH NH 3 Other acid halide reactions: O O CH 3 OMe O OH H2O Me2CuLi MeOH (pyr) (pyr) O OH O CH3 Li(tBuO)3AlH H CH3 1. 2eq MeMgBr Cl + O 2. H3O wkup Et2NH O O O (2 eq) O 2 eq NH3 O O NEt2 NH2 +NH Cl 4 Anhydrides Made from the acid chloride. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

The Acetylation of Naphthalene and the Formation
THE ACETYLATION OF NAPHTHALENE AND THE FORMATION OF (CHLOROVINYL)NAPHTHALENES THEREIN By GARY WARREN ~EEN Bachelor of Arts Southwestern College Winfield, Kansas 1963 Master of Science Oklahoma State University Stillwater, Oklahoma 1968 Submitted to the Faculty of the Graduate College of the Oklahoma State University in partial fulfillment of the requirements for the Degree of DOCTOR OF PHILOSOPHY July, 1976 THE ACETYLATION OF NAPHTHALENE AND THE FORMATION OF (CHLOROVINYL)NAPHTHALENES THEREIN Thesis Approved: 96419~~ ii ACKNOWLEDGMENTS I am especially grateful for the counseling, encouragement, artd technical assistance by Dr. E. J. Eisenbraun, my research adviser, throughout my graduate studies. I thank my co-workers at Conoco for their assistance. Much of this work would not have been possible without instruments which were con~tructed or modified by D. L. Winter. Lennis Hall has provided typing during the preparation of this thesis and other manuscripts, The assistance and encouragement of M. C. Hamming is gratefully acknowledged. I appreciate my supervisors at Conoco, especially H. T, Ford, J. C. Kirk, G. Perkins, D. B. Burrows and R. M. Tillman, for their encouragement and support during this research, Finally, I extend my gratitude to my wife Melva and my children, David, Steven and Traci, for the sacrifices they made over the last few years. iii TABLE OF CONTENTS Chapter Page I. INTRODUCTION. 1 Mass Fragmentography 4 II. RESULTS AND DISCUSSION .• 7 Acetylation of Naphthalene with Acetyl Chloride in Chloroform. • . 7 Analysis of Reaction Mixtures to Show the Formation of -4 and -5 10 III. EXPERIMENTAL. 16 Acetylation of Naphthalene with Acetyl Chloride- Aluminum Chloride in Chloroform. -

Acetyl Chloride As a Reagent for the Determination of Hydroxyl Groups
_.AC ETYL c-HLORIDE AS A REAGENt!' FOR THE DETEHM IN.A,.TION OF HYDROXYL GROUPS by LLOYD DREW PENNINGTON A THES IS submitted to the OREGON STATE COLLEGE in partial fulfillment of the requirements for the degree of MASTER OF ARTS June 1941 APPBIIED: Redacted for Privacy Aaal.atent Profeesor of CFmletry In Charge of llaJor Redacted for Privacy Eead of Departnent of Cbentetny Redacted for Privacy Chal.ruan of Sohool Gnaduate Conr{ttoe Redacted for Privacy Cbalman of State Collcge Ghraduate Counoll Acknowledgments I wish to express my thanks for the generous assist ance end advice given me by Dr. Bert E. Christensen. I also wish to thank Keene Dimick for his help in purification of some of the alcohols. Table of Contents Part I. Theory and Development Introduction . • • • • • • • • • • • • 1 Experimental • • • • • • • • • • • • • 2 Results end Discussions • • • • • • • • • 6 Summary. • • • • • • • • • • • • 10 Part II. Practical Application The Determination of Menthol in Oil of Pepp ermint. 11 Experimental • • • • • • • • • • • • • 12 Results and Discussion. • • • • • • • • • 13 Summary. • • • • .. • • • • • • • • 15 Tables Figure 1 and Figure 2 • • • • • • • • • • 5 Table I. • • • .. • • • • • • • • • • 7-9 Table II • • • • • • • • • • • • • 14 Table III . • 16 ' • • • • • • • • • • ACETYL CHLORIDE AS A REAGENT FOR THE DEI'ERMIN ATION OF HYDROXYL GROUPS Part I. Theory and Development Introduction The · simplest methods ~or the determination of the hydroxyl radical in organic compounds are those based on esterification procedures. Acetic anhydride with and without pyridine has been widely used in this connection (3, 4, 5, 7). Although acetyl chloride is a much more active acetylating agent little attention has been given to the possibility of its use in quantitative work (6). -

United States Patent (19) 11 Patent Number: 5,672,749
US005672749A UnitedO States Patent (19) 11 Patent Number: 5,672,749 Waites et al. (45) Date of Patent: Sep. 30, 1997 54 PROCESS FOR PREPARING ACETYL 2006,335 7/1935 Conover .................................. 562/863 CHLORDE 2,475,966 7/1949 Hull ........ ... 562/863 3,576,860 4/1971 Zazaris .................................... 562/863 75) Inventors: W. Bryan Waites, St. Matthews; Robert E. Young, West Columbia; FOREIGN PATENT DOCUMENTS Philip R. DeVrou, Orangeburg, all of 901598 5/1972 Canada. S.C. 27-4567 11/1952 Japan. 458541 1/1975 U.S.S.R. 73) Assignee: Albemarle Corporation, Richmond, Va. Primary Examiner-Paul J. Killos Attorney, Agent, or Firm-Philip M. Pippenger 21 Appl. No.: 631,295 57 ABSTRACT 22 Filed: Apr. 9, 1996 The yield of acetyl chloride from the reaction between acetic (51) Int. Cl. ... CO7C 51/58 anhydride and hydrogen chloride is increased by withdraw 52 U.S. Cl. ..................................................... 562/863 ing acetyl chloride, optionally together with at least some of 58) Field of Search ............................................... 562/863 the acetic acid by-product, from the reaction mixture as the reaction proceeds and recycling the remainder of the reac 56) References Cited tion mixture to the reactor for reaction with additional hydrogen chloride. U.S. PATENT DOCUMENTS 1,850,205 3/1932 Hale et al. .............................. 562/863 4 Claims, No Drawings 5,672,749 1 2 PROCESS FOR PREPARING ACETYL hydrogen chloride in addition to the acetic anhydride and CHLORDE acetic acid) or an acetic anhydride that has been used to absorb/capture excess HCl from a previous reaction. FIELD OF INVENTION When this technique is used, the feed rates are not critical 5 as long as they ensure contact with the acetic anhydride of This invention relates to a process for preparing acetyl a stoichiometric sufficiency of the hydrogen chloride. -

Chem263 Nov 25 Notes 2010
Chem 263 Nov 25, 2010 Preparation of Acid Chlorides Acid chlorides are prepared from carboxylic acids by reaction with thionyl chloride (SOCl2) by a mechanism we will not discuss now. O O SOCl 2 SO2 HCl R OH R Cl Hydrolysis of Acid Chlorides Acid chlorides react under acidic or basic conditions to yield carboxylic acids. This hydrolysis reaction is a typical nucleophilic acyl substitution. Acid halides, anhydrides, esters and amides react in a similar fashion under these hydrolysis conditions. Under basic conditions, the hydroxide anion attacks the carbonyl group of the acid chloride to yield a tetrahedral intermediate reversibly, which then displaces the chloride irreversibly to yield the carboxylic acid. Since we are under basic conditions, the base will deprotonate the acid to yield the carboxylate salt. The displacement step of the reaction is irreversible because the chloride is a far better atom to stabilize a negative charge than the oxygen. O O O O NaOH NaOH Cl- + R Cl R Cl R OH R O Na OH OH Under acidic conditions, the protonated carbonyl group of the acid chloride is attacked by water to yield the tetrahedral intermediate, which is then deprotonated to become a neutral species. The intermediate then displaces the chloride anion to yield the carboxylic acid. Once again the displacement step of the reaction is irreversible due to the reasons given above. H H H O O O H+ O R Cl R Cl HCl R Cl R OH OH OH H HO H Reactivity of Carboxylic acid Derivatives Nucleophilic acyl substitution reactions usually take place in two steps: addition of the nucleophile and elimination of a leaving group.