Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The Radiochemistry of Beryllium
National Academy of Sciences National Research Council I NUCLEAR SCIENCE SERIES The Radiochemistry ·of Beryllium COMMITTEE ON NUCLEAR SCIENCE L. F. CURTISS, Chairman ROBLEY D. EVANS, Vice Chairman National Bureau of Standards MassaChusetts Institute of Technol0gy J. A. DeJUREN, Secretary ./Westinghouse Electric Corporation H.J. CURTIS G. G. MANOV Brookhaven National' LaboratOry Tracerlab, Inc. SAMUEL EPSTEIN W. WAYNE MEINKE CalUornia Institute of Technology University of Michigan HERBERT GOLDSTEIN A.H. SNELL Nuclear Development Corporation of , oak Ridge National Laboratory America E. A. UEHLING H.J. GOMBERG University of Washington University of Michigan D. M. VAN PATTER E.D.KLEMA Bartol Research Foundation Northwestern University ROBERT L. PLATZMAN Argonne National Laboratory LIAISON MEMBERS PAUL C .. AEBERSOLD W.D.URRY Atomic Energy Commission U. S. Air Force J. HOW ARD McMILLEN WILLIAM E. WRIGHT National Science Foundation Office of Naval Research SUBCOMMITTEE ON RADIOCHEMISTRY W. WAYNE MEINKE, Chairman HAROLD KIRBY University of Michigan Mound Laboratory GREGORY R. CHOPPIN GEORGE LEDDICOTTE Florida State University. Oak Ridge National Laboratory GEORGE A. COW AN JULIAN NIELSEN Los Alamos Scientific Laboratory Hanford Laboratories ARTHUR W. FAIRHALL ELLIS P. STEINBERG University of Washington Argonne National Laboratory JEROME HUDIS PETER C. STEVENSON Brookhaven National Laboratory University of California (Livermore) EARL HYDE LEO YAFFE University of CalUornia (Berkeley) McGill University CONSULTANTS NATHAN BALLOU WILLIAM MARLOW Naval Radiological Defense Laboratory N atlonal Bureau of Standards JAMESDeVOE University of Michigan CHF.MISTRY-RADIATION AND RADK>CHEMIST The Radiochemistry of Beryllium By A. W. FAIRHALL. Department of Chemistry University of Washington Seattle, Washington May 1960 ' Subcommittee on Radiochemistry National Academy of Sciences - National Research Council Printed in USA. -

B.Sc.(H) Chemistry-3Rd Semester
LIBRARY (18 [This question paper contains 4 printed pages Your Roll No. S1. No. of Q. Paper :7393 J Unique Paper Code 32171301 Name of the Course : B.Sc.(Hons.) Chemistry Name of the Paper Inorganic Chemistry II: s and p block elements Semester : II Time: 3 Hours Maximum Marks : 75 Instructions for Candidates: (i) Write your Roll No.. on the top immediately on receipt of this question paper. (ii) Attempt any five questions. (iii) All questions carry equal marks. 1. (a) Explain why most lines in the Ellingham diagram slope upward from left to right. What happens when a line crosses AG=0? 5 (b) Why is white phosphorus very reactive in comparison to red phosphorus ? Give the mechanism of stepwise hydrolysis of P,O,a. P.T.O 7393 7393 in Discuss the structure and bonding obtain the following: (c) formed (c) How will you Diborane. What are the products borazine ammonia (i) B-bromoborazine from when diborane reacts with excess 5 (ii) (NPF,), from (NPCl,), at (i) low temperature Lithium is different from other 2. (a) Chemistry of (ii) high temperature of alkali metals. Give examples in support 5 3515 the statement. 4. Give reason (any five): the gases? more stable than P, (b) What are clathrate compounds of noble (i) P, molecule is clathrates? Why do helium and neon not form molecule. 5 from B to Al but (ii) lonization energy decreases Ga. of increases from Al to Give one method of preparation (c) but a gas at room is the a liquid H,S peroxodisulphuric acid. -

Chlorine Substituted Acetic Acids and Salts. Effect of Salification on Chlorine-35 NQR*
Chlorine Substituted Acetic Acids and Salts. Effect of Salification on Chlorine-35 NQR* Serge David3, Michel Gourdjib, Lucien Guibéb, and Alain Péneaub a Laboratoire de Chimie Organique Multifonctionnelle, Bätiment 420. b Institut d'Electronique Fondamentale, Bätiment 220, Universite Paris-Sud, 91405 ORSAY Cedex, France. Z. Naturforsch. 51a, 611-619 (1996); received October 10, 1995 The NQR of a quadrupolar probe nucleus is often used to investigate the effect of substituent in molecules. The inductive effect, based on a partial charge migration along the molecular skeleton is the only one present in saturated aliphatics, the conjugative effect appearing in conjugated molecules, especially aromatics. As the stepwise charge migration mechanism, formerly used to explain the inductive effect, is now believed obsolete, we have wanted to reexamined the case of chlorine substituted acetic acids and salts. The data in literature was extended by observing reso- nances and determining NQR frequencies in several acids and salts. The present analysis of the salification of mono-, di- and tri-chloroacetic acids, which is equivalent to a deprotonation or the substitution of the acid hydrogen by a negative unit charge, shows that a model based on the polarization of the chlorine atom(s) by the carboxyle group is consistent with experimental results: the polarization energy appears to be proportional to the NQR frequency shifts; experimental data show a correlation between the NQR frequency shifts accompanying salification and the variations of the intrinsic acidity measured in the gas phase; this, in turn shows that there is a proportionality between the polarization energy and the variations in the acid free enthalpy of dissociation. -
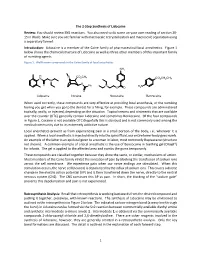
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Monochloroacetic Acid, Sodium Monochloroacetate
Monochloroacetic acid, sodium monochloroacetate MAK Value Documentation, supplement – Translation of the German version from 2019 A. Hartwig1,* MAK Commission2,* 1 Chair of the Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds Keywords: in the Work Area, Deutsche Forschungsgemeinschaft, Institute of Applied Biosciences, Department of Food Chemistry and Toxicology, Karlsruhe Institute of Technology (KIT), Adenauerring 20a, Building 50.41, monochloroacetic acid, sodium 76131 Karlsruhe, Germany monochloroacetate, irritation, 2 Permanent Senate Commission for the Investigation of Health Hazards of Chemical Compounds in the Work oxidative stress, peak limitation, Area, Deutsche Forschungsgemeinschaft, Kennedyallee 40, 53175 Bonn, Germany skin absorption, maximum workplace concentration, MAK * E-Mail: A. Hartwig ([email protected]), MAK Commission ([email protected]) value, prenatal toxicity, hazardous substance Abstract The German Commission for the Investigation of Health Hazards of Chemical Com- pounds in the Work Area has re-evaluated monochloroacetic acid [79-11-8] together with sodium monochloroacetate [3926-62-3] considering all toxicological endpoints. Monochloroacetic acid is a strong acid and dissociates in biological media. Therefore, also data for sodium monochloroacetate are used to evaluate the systemic toxicity. Monochloroacetic acid is corrosive to the eyes but there are no inhalation studies from which a NOAEC for local effects can be derived. Therefore, a maximum con- centration at the workplace (MAK value) of 2 mg/m3 (0.5 ml/m3), which has been set for the better investigated phosphoric acid, is also established for monochloroacetic acid.Asirritationisthecriticaleffect,monochloroaceticacidisclassifiedinPeakLim- Citation Note: itation Category I. By analogy with phosphoric acid, an excursion factor of 2 is set. Hartwig A, MAK Commission. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

Ester Resveratrol Analogues, Chromium Trioxide Oxidation of Terpenes, and Synthesis of Mimics of (-)-Englerin A
Brigham Young University BYU ScholarsArchive Theses and Dissertations 2014-08-01 Synthesis of 4'-Ester Resveratrol Analogues, Chromium Trioxide Oxidation of Terpenes, and Synthesis of Mimics of (-)-Englerin A Mark Jeffrey Acerson Brigham Young University - Provo Follow this and additional works at: https://scholarsarchive.byu.edu/etd Part of the Biochemistry Commons, and the Chemistry Commons BYU ScholarsArchive Citation Acerson, Mark Jeffrey, "Synthesis of 4'-Ester Resveratrol Analogues, Chromium Trioxide Oxidation of Terpenes, and Synthesis of Mimics of (-)-Englerin A" (2014). Theses and Dissertations. 5458. https://scholarsarchive.byu.edu/etd/5458 This Dissertation is brought to you for free and open access by BYU ScholarsArchive. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of BYU ScholarsArchive. For more information, please contact [email protected], [email protected]. Synthesis of 4’-Ester Resveratrol Analogues, Chromium Trioxide Oxidation of Terpenes, and Synthesis of Mimics of (–)-Englerin A Mark Jeffrey Acerson A dissertation submitted to the faculty of Brigham Young University in partial fulfillment of the requirements for the degree of Doctor of Philosophy Merritt B. Andrus, Chair Steven L. Castle Matt A. Peterson Joshua L. Price Richard K. Watt Department of Chemistry and Biochemistry Brigham Young University August 2014 Copyright © 2014 Mark Jeffrey Acerson All Rights Reserved ABSTRACT Synthesis of 4’-Ester Resveratrol Analogues, Chromium Trioxide Oxidation of Terpenes, and Synthesis of Mimics of (–)-Englerin A Mark Jeffrey Acerson Department of Chemistry and Biochemistry, BYU Doctor of Philosophy 4’-ester analogues of resveratrol were synthesized using reaction conditions developed to produce mono-ester products in the presence of two other unprotected phenols. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

Dehydrogenation of Ethanol to Acetaldehyde Over Different Metals Supported on Carbon Catalysts
catalysts Article Dehydrogenation of Ethanol to Acetaldehyde over Different Metals Supported on Carbon Catalysts Jeerati Ob-eye , Piyasan Praserthdam and Bunjerd Jongsomjit * Center of Excellence on Catalysis and Catalytic Reaction Engineering, Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University, Bangkok 10330, Thailand; [email protected] (J.O.-e.); [email protected] (P.P.) * Correspondence: [email protected]; Tel.: +66-2-218-6874 Received: 29 November 2018; Accepted: 27 December 2018; Published: 9 January 2019 Abstract: Recently, the interest in ethanol production from renewable natural sources in Thailand has been receiving much attention as an alternative form of energy. The low-cost accessibility of ethanol has been seen as an interesting topic, leading to the extensive study of the formation of distinct chemicals, such as ethylene, diethyl ether, acetaldehyde, and ethyl acetate, starting from ethanol as a raw material. In this paper, ethanol dehydrogenation to acetaldehyde in a one-step reaction was investigated by using commercial activated carbon with four different metal-doped catalysts. The reaction was conducted in a packed-bed micro-tubular reactor under a temperature range of 250–400 ◦C. The best results were found by using the copper doped on an activated carbon catalyst. Under this specified condition, ethanol conversion of 65.3% with acetaldehyde selectivity of 96.3% at 350 ◦C was achieved. This was probably due to the optimal acidity of copper doped on the activated carbon catalyst, as proven by the temperature-programmed desorption of ammonia (NH3-TPD). In addition, the other three catalyst samples (activated carbon, ceria, and cobalt doped on activated carbon) also favored high selectivity to acetaldehyde (>90%). -

ACETIC ACID and ACETIC ANHYDRIDE (November 1994)
Abstract Process Economics Program Report 37B ACETIC ACID AND ACETIC ANHYDRIDE (November 1994) This Report presents preliminary process designs and estimated economics for the manufacture of acetic acid and acetic anhydride by carbonylation technology. The three processes evaluated in this report include Monsanto’s low pressure carbonylation of methanol process (BP Chemical acquired licensing rights to this process in 1985), Eastman’s process for carbonylation of methyl acetate to produce acetic anhydride (methanol added to the reaction mixture results in the coproduction of acetic acid in this process), and a process based on BP Chemical patents that coproduces acetic acid and acetic anhydride via carbonylation of methyl acetate in the presence of water. Both the Eastman and BP Chemical processes are back– integrated into the manufacture of the methyl acetate feedstock from methanol and acetic acid. We have included a discussion of other commercialized acetic acid and acetic anhydride processes as well as potential new processes. A list of the world’s acetic acid and acetic anhydride producers along with their estimated plant capacities and a description of the major acetic acid and acetic anhydride markets are also included in this Report. This Report will be useful to producers of acetic acid and acetic anhydride, as well as to producers of methanol and downstream products such as vinyl acetate monomer. PEP’93 MKG CONTENTS 1 INTRODUCTION 1-1 2 SUMMARY 2-1 GENERAL ASPECTS 2-1 ECONOMIC ASPECTS 2-1 TECHNICAL ASPECTS 2-3 Low Pressure Carbonylation -

Acetic Anhydride
ACETIC ANHYDRIDE Method number: 102 Matrix: Air Target concentration: 5 ppm (20 mg/m3) OSHA PEL: 5 ppm (20 mg/m3) TWA ACGIH TLV: 5 ppm (20 mg/m3) ceiling Procedure: Samples are collected open face on glass fiber filters coated with veratrylamine and di-n-octyl phthalate. Samples are extracted with 50/50 (v/v) 2-propanol/toluene and analyzed by GC using a nitrogen- phosphorus detector (NPD). Recommended air volume and sampling rate: 7.5 L at 0.5 L/min ceiling 7.5 L at 0.05 L/min TWA Reliable quantitation limit: 0.094 ppm (0.39 mg/m3) Standard error of estimate at the target concentration: 6.4% Special caution: Ketene and acetyl chloride produce the same derivative as acetic anhydride. Coated filters should be used within a month of preparation. Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: October 1993 Chemist: Yihlin Chan Organic Methods Evaluation Branch OSHA Salt Lake Technical Center Salt Lake City, UT 84165-0200 1. General Discussion 1.1 Background 1.1.1 History In OSHA Method 82, acetic anhydride is collected on a glass fiber filter impregnated with 1-(2-pyridyl)piperazine, which reacts with the anhydride to form a derivative (Ref. 5.1). Attempts at using 1-(2-pyridyl)piperazine for the derivatization of maleic, phthalic, and trimellitic anhydrides failed, however, because the resulting derivatives of these anhydrides were found to be unstable. These anhydrides were derivatized with veratrylamine instead (Refs. 5.2-5.4).