SYNTHETIC METHODS General Methods Compounds Were Synthesized Using Standard Procedures
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

An Integrated Flow and Batch-Based Approach for the Synthesis of O-Methyl Siphonazole Amarcus Combined Flow and Batch Synthesis of O-Methyl Siphonazole Baumann, Ian R
LETTER 1375 An Integrated Flow and Batch-Based Approach for the Synthesis of O-Methyl Siphonazole AMarcus Combined Flow and Batch Synthesis of O-Methyl Siphonazole Baumann, Ian R. Baxendale, Malte Brasholz, John J. Hayward, Steven V. Ley, Nikzad Nikbin Innovative Technology Centre, Department of Chemistry, University of Cambridge, Lensfield Road, Cambridge CB2 1EW, UK E-mail: [email protected] Received 21 February 2011 has transformed flow chemistry from an academic interest Abstract: The bisoxazole containing natural product O-methyl si- phonazole was assembled using a suite of microreactors via a flow- to a valuable enabling technology that overcomes several based approach in concert with traditional batch methods. The use of the bottlenecks traditionally faced by synthetic chem- 6 of a toolbox of solid-supported scavengers and reagents to aid puri- ists. As such the generation and immediate use of hazard- fication afforded the natural product in a total of nine steps. ous, toxic, or unstable intermediates7 has been reported as Key words: oxazoles, Claisen condensation, flow chemistry, well as the possibility to more reliably perform reaction microreactors, solid-supported reagents scale-up.8 Furthermore, flow reactors can be transformed into automated platforms by the addition of liquid han- dling and fraction collector modules expanding the work- As part of our ongoing interest in oxazole-containing nat- ing capabilities of the units and allowing for 24/7 working 9 ural products,1 we have devised a synthetic route to the regimes. Finally, individual reactions can be telescoped bisoxazole alkaloid O-methyl siphonazole (2), which was into one continuous flow sequence, thus avoiding the iter- discovered by König and co-workers in 2006, along with ative isolation, purification, and reprocessing of interme- 10 the C-21-demethylated parent compound siphonazole (1, diates. -

5 Phosphorus Oxychloride1 Acute Exposure Guideline Levels
Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 10 Committee on Acute Exposure Guideline Levels Committee on Toxicology Board on Environmental Studies and Toxicology Division on Earth and Life Studies Copyright © National Academy of Sciences. All rights reserved. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 10 THE NATIONAL ACADEMIES PRESS 500 FIFTH STREET, NW WASHINGTON, DC 20001 NOTICE: The project that is the subject of this report was approved by the Governing Board of the National Research Council, whose members are drawn from the councils of the National Academy of Sciences, the National Academy of Engineering, and the Insti- tute of Medicine. The members of the committee responsible for the report were chosen for their special competences and with regard for appropriate balance. This project was supported by Contract No. W81K04-06-D-0023 and EP-W-09-007 be- tween the National Academy of Sciences and the U.S. Department of Defense and the U.S. Environmental Protection Agency. Any opinions, findings, conclusions, or recom- mendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the organizations or agencies that provided support for this project. International Standard Book Number-13: 978-0-309-21987-7 International Standard Book Number-10: 0-309-21987-6 Additional copies of this report are available from The National Academies Press 500 Fifth Street, NW Box 285 Washington, DC 20055 800-624-6242 202-334-3313 (in the Washington metropolitan area) http://www.nap.edu Copyright 2011 by the National Academy of Sciences. -
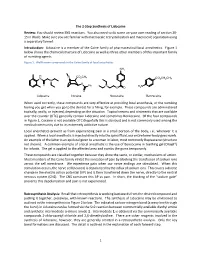
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

1. A. the First Reaction Is a Friedel-Crafts Acylation (FCA), Where the Major Product Is the Para- Isomer (60% Isolated Yield)
1. a. The first reaction is a Friedel-Crafts Acylation (FCA), where the major product is the para- isomer (60% isolated yield). The second reaction is a nitration, where the incoming electrophile (nitronium ion) is directed to the ortho position of the methoxy group. The last reaction is a Wolff-Kishner reduction that converts the acetyl group into an ethyl group. The nitro group does not react under these conditions. OCH OCH OCH OCH3 3 3 3 NO2 NO2 N2H4/KOH CH3COCl/AlCl3 H2SO4/HNO3 0 oC O CH 3 O CH3 CH3 (A) Reaction 1 (B) Reaction 2 (C) Reaction 3 (P) b. The best solvent for the FC-acylation is dichloromethane. Tetrahydrofuran is a fairly strong Lewis base, which would react and deactivate the AlCl3 catalyst. Ethanol would also react with AlCl3 and form alcoholates, which are inactive at FCA catalyst. Dichloromethane is polar enough to dissolve all three compounds but does not form adducts with AlCl3. Thus, aluminum chloride maintains its Lewis acidity. 3+ c. As discussed in lecture, AlCl3*6 H2O is not suitable as catalyst because the Al is not a strong Lewis acid anymore. In addition, larger amounts of water would destroy the acetyl chloride as well (=hydrolysis, CH3COCl + H2O ---- > CH3COOH + HCl). Consequently, the reaction would not proceed in the desired fashion. 3+ OH2 H2O OH2 Al H2O OH2 OH2 d. In order to determine the yield, one has to calculate the number of moles of the reactant and the product. nA = 1.90 mL * 0.996 g/mL/108.14 g/mol = 17.5 mmol nCH3COCl = 2.49 mL * 1.104 g/mL/78.5 g/mol = 35.0 mmol nAlCl3 = 4.67 g/133.5 g/mol = 35.0 mmol Compound (A) is the limiting reagent. -

United States Patent (19) (11) 4,129,595 Suzuki 45) Dec
United States Patent (19) (11) 4,129,595 Suzuki 45) Dec. 12, 1978 (54) PREPARATION OF CHLOROACETYL (56) References Cited CHLORDE PUBLICATIONS E. E. Blaise et al., Comptes Rendus (France), vol. 174, 75 Inventor: Shigeto Suzuki, San Francisco, Calif. pp. 1173-1174, (1922), (Chem. Abstr., vol. 16, 2480). 73) Assignee: Chevron Research Company, San Primary Examiner-Gerald A. Schwartz Francisco, Calif. Attorney, Agent, or Firm-D. A. Newell; John Stoner, Jr. (21) Appl. No.: 891,429 57 ABSTRACT Chloroacetyl chloride is prepared by reacting glycolic (22) Filed: Mar. 29, 1978 acid with thionyl chloride in the presence of nitrogen 51) int. C.’.............................................. CO7C 51/58 containing organic compound orphosphine compound. 52 U.S. C. ................................................ 260/544 Y 58 Field of Search .................................... 260/544 Y 3 Claims, No Drawings 4,129,595 1. 2 glycolic acid with thionyl chloride in the presence of a PREPARATION OF CHLOROACETYL CHLORDE catalytic amount of nitrogen-containing hydrocarbyl organic compound or hydrocarbyl phosphine com BACKGROUND OF THE INVENTION pound at high conversion and yield in accordance with 1. Field of the Invention 5 the present invention. The present invention relates to the preparation of chloroacetyl chloride. More particularly, the invention DETALED DESCRIPTION OF THE relates to the preparation of chloroacetyl chloride by INVENTION reacting glycolic acid with thionyl chloride in the pres The nitrogen-containing hydrocarbyl organic com ence of nitrogen-containing -

Ketene Reactions. I. the Addition of Acid Chlorides
KETENE REACTIONS. I. THE ADDITION OF ACID CHLORIDES TO DIMETHYLKETENE. II. THE CYCLOADDITION OF KETENES TO CARBONYL COMPOUNDS APPROVED: Graduate Committee: Major Professor Committee Member.rr^- Committee Member Committee Member Director of the Department of Chemistry Dean' of the Graduate School Smith, Larry, Ketene Reactions. I. The Addition of Acid Chlorides to DimethyIketene. II. The Cycloaddition of Ketenes to Carbonvl Compounds. Doctor of Philosophy (Chemistry), December, 1970, 63 pp., 3 tables, bibliography, 62 titles. Part I describes the addition of several acid chlorides to dimethylketene. The resulting 3-ketoacid chlorides were isolated and characterized. The reactivities of acid chlorides were found to parallel the parent acid pKa's. A reactivity order of ketenes toward acid chlorides was established. Dimethylketene is more reactive than ketene which is more reactive than diphenylketene. Attempts to effect the addition of an acid halide to a ketene produced by in situ dehydro- halogenation yielded a-halovinyl esters. The addition of acid chlorides to ketenes was concluded to be an ionic process dependent upon the nucleophilic character of the ketene oc- carbon and the polarity of the carbon-chlorine bond in the acid chloride. Part II describes the cycloaddition of several aldo- ketenes to chloral. The ketenes were generated in situ by dehydrohalogenation and dehalogenation of appropriately substituted acyl halides. Both cis- and trans-4-trichloro- Miyl-2-oxetanones are produced in the cycloadditions with the sterically hindered cis isomer predominating. Isomer distributions were determined by vpc or nmr analysis of the reaction solutions. Production of the ketenes by dehalo- genation resulted in enhanced reactivity of the carbonyl compounds. -

Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid
Asian Journal of Chemistry; Vol. 26, No. 2 (2014), 475-480 http://dx.doi.org/10.14233/ajchem.2014.15484 Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid * JIAN-WEI XUE , JIAN-PENG ZHANG, BO WU, FU-XIANG LI and ZHI-PING LV Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, P.R. China *Corresponding author: Fax: +86 351 6111178; Tel: +86 351 60105503; E-mail: [email protected] Received: 14 March 2013; Accepted: 17 May 2013; Published online: 15 January 2014; AJC-14570 The process of acetic acid catalysis and chlorination for synthesizing chloroacetic acid can exist in not only gas phase but also liquid phase. In this paper, the gas-phase reaction mechanism of the synthesis of chloroacetic acid was studied. Due to the high concentration of acetic acid and the better reaction mass transfer in the liquid-phase reaction, the generation amount of the dichloroacetic acid was higher than that in the gas-phase reaction. Under the solution distillation, the concentration of acetyl chloride, whose boiling point is very low, was very high in the gas phase, sometimes even up to 99 %, which would cause the acetyl chloride to escape rapidly with the hydrogen chloride exhaust, so that the reaction slowed down. Therefore, series reactions occured easily in the gas-phase reaction causing the amount of the dichloroacetic acid to increase. Keywords: Gas phase, Catalysis, Chlorination, Chloroacetic acid, Acetic acid. INTRODUCTION Martikainen et al.3 summed up the reaction mechanism that was consistent with a mechanism found by Sioli according Chloroacetic acid is not only a fine chemical product but to the system condition experiment and systematic theoretical also an important intermediate in organic synthesis. -

Halogenation Reagents
Halogenation Reagents Halogenation is a basic and fundamental transformation in organic chemistry, and halogenated compounds are of extreme importance as building blocks in organic synthesis. The development of modern coupling reactions, such as the [P2140] Suzuki-Miyaura and Mizoroki-Heck reactions, have greatly increased the demand for halogenated compounds as starting materials. P2140 (2.3 eq.) On the other hand, introduction of fluorine into a certain position of bioactive compound such as a pharmaceutical and an agricultural chemical may remarkably reduce the toxicity of the compound, or improve the efficiency of medicine. This is due to the structurally mimic and blocking effect characterized by fluorine. P2140 (3 eq.) In response to this situation, a number of novel halogenation reagents have been developed. 4-tert-Butyl-2,6-dimethylphenylsulfur trifluoride (FLUOLEAD™) [B3664] is introduced as below: B3664 is a novel nucleophilic 1-Fluoro-3,3-dimethyl-1,2-benziodoxole [F0957] is a hypervalent fluorinating agent which was first reported by Umemoto et al.1) iodine derivative developed by Stuart et al.3) F0957 is stable to air Differing from other existing fluorinating agents, such as DAST, and moisture and used as an electrophilic fluorinating reagent for B3664 is a crystalline solid with high thermal stability and less a α-monofluorination of β-ketoesters in the presence of fuming character, which makes it easier to handle. B3664 triethylamine trihydrofluoride. fluorinates a hydroxyl or carbonyl group to afford the corresponding fluorinated compounds in good yields.1) F I O [F0957] O O Ph OEt F0957(2eq.) F O O Et3N-3HF(2.7eq.) [B3664] Ph OEt CH2Cl2 O O 40oC,24h Ph OEt F F Dibromoisocyanuric acid (DBI) [D3753] which was first reported by Gottardi, is a mild and highly effective brominating agent,4a,b,c) and has superior brominating ability when compared with N-bromosuccinimide (NBS), which is frequently used in organic IF5-Pyridine-HF (Hara Reagent) [P2140] is also a novel synthesis. -

Phosphonate–Phosphonochloridate Conversion Bogdan Iorga, Duncan Carmichael, Philippe Savignac
Phosphonate–phosphonochloridate conversion Bogdan Iorga, Duncan Carmichael, Philippe Savignac To cite this version: Bogdan Iorga, Duncan Carmichael, Philippe Savignac. Phosphonate–phosphonochloridate conversion. Comptes rendus de l’Académie des sciences. Série IIc, Chimie, Elsevier, 2000, 3 (11-12), pp.821-829. 10.1016/s1387-1609(00)01207-x. hal-03161486 HAL Id: hal-03161486 https://hal.archives-ouvertes.fr/hal-03161486 Submitted on 10 Mar 2021 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Phosphonate-Phosphonochloridate Conversion Bogdan Iorga, Duncan Carmichael, Philippe Savignac Laboratoire Hétéroéléments et Coordination, UMR CNRS 7653, DCPH, Ecole Polytechnique, 91128 Palaiseau Cedex, France; E-mail : [email protected] __________________________________________________________________________________________ Abstract - This review deals with the phosphonate-phosphonochloridate conversion. The different phosphorus-chlorine bond forming reagents (COCl2, (COCl)2, SOCl2, PCl5, POCl3, Ph3PCl2, trichloro(o-phenylenedioxy)phosphorane) -

European Patent Office Office Europeenpeen Des Brevets EP 0 674 618 B1
Europaisches Patentamt (19) European Patent Office Office europeenpeen des brevets EP 0 674 618 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) intci.6: C07C 317/32, C07C 233/22, of the grant of the patent: C07C 315/04, C07D 301/19, 09.09.1998 Bulletin 1998/37 C07D 263/14, A61K31/16 (21) Application number: 94903599.2 (86) International application number: PCT/US93/12071 (22) Date of filing: 15.12.1993 (87) International publication number: WO 94/14764 (07.07.1994 Gazette 1994/15) (54) ASYMMETRIC PROCESS FOR PREPARING FLORFENICOL, THIAMPHENICOL, CHLORAMPHENICOL AND OXAZOLINE INTERMEDIATES ASYMMETRISCHES HERSTELLUNGSVERFAHREN FUR FLORFENICOL, THIAMPHENICOL, CHLORAMPHENICOL UND OXAZOLIN-ZWISCHENPRODUKTE PROCEDE ASYMETRIQUE DE PREPARATION DE FLORFENICOL, THIAMPHENICOL, CHLORAMPHENICOL ET D'INTERMEDI AIRES OXAZOLINE (84) Designated Contracting States: (74) Representative: AT BE CH DE DK ES FR GB GR IE IT LI LU NL PT von Kreisler, Alek, Dipl.-Chem. et al SE Patentanwalte, von Kreisler-Selting-Werner, (30) Priority: 18.12.1992 US 993932 Bahnhofsvorplatz 1 (Deichmannhaus) 50667 Koln (DE) (43) Date of publication of application: 04.10.1995 Bulletin 1995/40 (56) References cited: EP-A- 0 423 705 EP-A- 0 472 790 (73) Proprietor: SCHERING CORPORATION WO-A-92/07824 US-A- 4 235 892 Kenilworth New Jersey 07033 (US) US-A- 4 876 352 US-A- 4 900 847 (72) Inventors: • CHEMICAL ABSTRACTS, vol. 107, no. 1, 06 July • WU, Guang-Zhong 1987, Columbus, Ohio, US; abstract no. 6859M, Somerville, NJ 08876 (US) JOMMI, GIANCARLO ET AL '2-Oxazolidinones • TORMOS, Wanda, I. as regioselective protection of beta-amino Elizabeth, NJ 07202 (US) alcohols in the synthesis of 2-amino-1-aryl-3-fluoro-1-propanols' page 632; column 1; & GAZZ.