Chloroacetyl Chloride
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
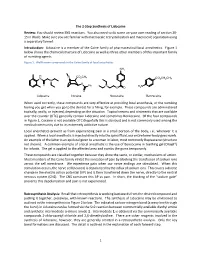
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

APPENDIX G Acid Dissociation Constants
harxxxxx_App-G.qxd 3/8/10 1:34 PM Page AP11 APPENDIX G Acid Dissociation Constants § ϭ 0.1 M 0 ؍ (Ionic strength ( † ‡ † Name Structure* pKa Ka pKa ϫ Ϫ5 Acetic acid CH3CO2H 4.756 1.75 10 4.56 (ethanoic acid) N ϩ H3 ϫ Ϫ3 Alanine CHCH3 2.344 (CO2H) 4.53 10 2.33 ϫ Ϫ10 9.868 (NH3) 1.36 10 9.71 CO2H ϩ Ϫ5 Aminobenzene NH3 4.601 2.51 ϫ 10 4.64 (aniline) ϪO SNϩ Ϫ4 4-Aminobenzenesulfonic acid 3 H3 3.232 5.86 ϫ 10 3.01 (sulfanilic acid) ϩ NH3 ϫ Ϫ3 2-Aminobenzoic acid 2.08 (CO2H) 8.3 10 2.01 ϫ Ϫ5 (anthranilic acid) 4.96 (NH3) 1.10 10 4.78 CO2H ϩ 2-Aminoethanethiol HSCH2CH2NH3 —— 8.21 (SH) (2-mercaptoethylamine) —— 10.73 (NH3) ϩ ϫ Ϫ10 2-Aminoethanol HOCH2CH2NH3 9.498 3.18 10 9.52 (ethanolamine) O H ϫ Ϫ5 4.70 (NH3) (20°) 2.0 10 4.74 2-Aminophenol Ϫ 9.97 (OH) (20°) 1.05 ϫ 10 10 9.87 ϩ NH3 ϩ ϫ Ϫ10 Ammonia NH4 9.245 5.69 10 9.26 N ϩ H3 N ϩ H2 ϫ Ϫ2 1.823 (CO2H) 1.50 10 2.03 CHCH CH CH NHC ϫ Ϫ9 Arginine 2 2 2 8.991 (NH3) 1.02 10 9.00 NH —— (NH2) —— (12.1) CO2H 2 O Ϫ 2.24 5.8 ϫ 10 3 2.15 Ϫ Arsenic acid HO As OH 6.96 1.10 ϫ 10 7 6.65 Ϫ (hydrogen arsenate) (11.50) 3.2 ϫ 10 12 (11.18) OH ϫ Ϫ10 Arsenious acid As(OH)3 9.29 5.1 10 9.14 (hydrogen arsenite) N ϩ O H3 Asparagine CHCH2CNH2 —— —— 2.16 (CO2H) —— —— 8.73 (NH3) CO2H *Each acid is written in its protonated form. -

United States Patent Office Patented Apr
3,576,860 United States Patent Office Patented Apr. 27, 1971 2 3,576,860 Further objects, aspects and advantages of the inven PREPARATION OF CHLOROACETYL CHLORIDE . tion will be apparent from the description which follows. Dimitri A. Zazaris, Belleville, Ill., assignor to Chlorination of acetic acid alone in the absence of a Monsanto Company, St. Louis, Mo. catalyst by prior art processes usually requires relatively No Drawing. Filed Dec. 20, 1967, Ser. No. 691,975 5 high temperatures, 110 to 120° C., in order for the re Int, C. C07c53/20 action to occur. Temperature in this range, however, re U.S. C. 260-539 9 Claims Sult in a high yield of dichloroacetic acid. In order to re duce the reaction temperature, acetic anhydride, acetyl chloride, inorganic salts or combination of the three have ABSTRACT OF THE DISCLOSURE O been added to the acetic acid. Reaction ompositions of 85 weight percent acetic anhydride and 15 weight percent Chlorination of acetic anhydride and acetic anhydride acetic acid have been utilized. In the chlorination of this acetic acid mixtures in the presence of lithium chloride to mixture, the product obtained was predominantly mono yield a mixture of monochloroacetyl chloride and mono chloroacetic acid. This is contrary to what would be ex chloroacetic acid having a small concentration of the poly pected since theoretically acetic anhydride should give 1 chlorinated derivatives. mole of acid and 1 mole of acid halide per mole of an hydride. The use of many inorganic salts did not mate rially change this result. Many of the present commercial herbicides, such as the It has previously been determined that the low yield of chloroacetamides and "2,4-D,' utilize a monochloroacetyl 20 monochloroacetyl chloride is due to the interactions of chloride or monochloroacetic acid as an intermediate. -

1. A. the First Reaction Is a Friedel-Crafts Acylation (FCA), Where the Major Product Is the Para- Isomer (60% Isolated Yield)
1. a. The first reaction is a Friedel-Crafts Acylation (FCA), where the major product is the para- isomer (60% isolated yield). The second reaction is a nitration, where the incoming electrophile (nitronium ion) is directed to the ortho position of the methoxy group. The last reaction is a Wolff-Kishner reduction that converts the acetyl group into an ethyl group. The nitro group does not react under these conditions. OCH OCH OCH OCH3 3 3 3 NO2 NO2 N2H4/KOH CH3COCl/AlCl3 H2SO4/HNO3 0 oC O CH 3 O CH3 CH3 (A) Reaction 1 (B) Reaction 2 (C) Reaction 3 (P) b. The best solvent for the FC-acylation is dichloromethane. Tetrahydrofuran is a fairly strong Lewis base, which would react and deactivate the AlCl3 catalyst. Ethanol would also react with AlCl3 and form alcoholates, which are inactive at FCA catalyst. Dichloromethane is polar enough to dissolve all three compounds but does not form adducts with AlCl3. Thus, aluminum chloride maintains its Lewis acidity. 3+ c. As discussed in lecture, AlCl3*6 H2O is not suitable as catalyst because the Al is not a strong Lewis acid anymore. In addition, larger amounts of water would destroy the acetyl chloride as well (=hydrolysis, CH3COCl + H2O ---- > CH3COOH + HCl). Consequently, the reaction would not proceed in the desired fashion. 3+ OH2 H2O OH2 Al H2O OH2 OH2 d. In order to determine the yield, one has to calculate the number of moles of the reactant and the product. nA = 1.90 mL * 0.996 g/mL/108.14 g/mol = 17.5 mmol nCH3COCl = 2.49 mL * 1.104 g/mL/78.5 g/mol = 35.0 mmol nAlCl3 = 4.67 g/133.5 g/mol = 35.0 mmol Compound (A) is the limiting reagent. -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 15-Feb-2011 Revision Date 10-Dec-2020 Revision Number 7 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Chloroacetic acid Cat No. : 108510000; 108510010; 108510025; 108510040; 108512500 Synonyms MCA CAS-No 79-11-8 EC-No. 201-178-4 Molecular Formula C2 H3 Cl O2 Reach Registration Number 01-2119459589-18 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Sector of use SU3 - Industrial uses: Uses of substances as such or in preparations at industrial sites Product category PC21 - Laboratory chemicals Process categories PROC15 - Use as a laboratory reagent Environmental release category ERC6a - Industrial use resulting in manufacture of another substance (use of intermediates) Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company UK entity/business name Fisher Scientific UK Bishop Meadow Road, Loughborough, Leicestershire LE11 5RG, United Kingdom EU entity/business name Acros Organics BVBA Janssen Pharmaceuticalaan 3a 2440 Geel, Belgium E-mail address [email protected] 1.4. Emergency telephone number For information US call: 001-800-ACROS-01 / Europe call: +32 14 57 52 11 Emergency Number US:001-201-796-7100 / Europe: +32 14 57 52 99 CHEMTREC Tel. No.US:001-800-424-9300 / Europe:001-703-527-3887 SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification -

Safety Data Sheet MCAA
SAFETY DATA SHEET MONOCHLOROACETIC ACID 80% - 90% AQ Solution Revision Date: September 2019 Previous date: May 2014 Print Date: September 2019 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1 Product information Commercial Product Name Monochloroacetic Acid Chemical Formula CICH2CO2H Molecular wt. 94.50 80-90% Solution 1.2 Relevant identified uses of the substance or mixture and uses advised against Use of the Substance/Mixture Recommended restrictions on use Reserved for industrial and professional use. 1.3 Details of the supplier of the safety data sheet Niacet Corporation 400 47th Street Niagara Falls, NY 14304 U.S.A. Telephone +1 716-285-1474 Telefax +1 716-285-1497 [email protected] 1.4 Emergency telephone number For Niacet Corporation, Niagara Falls, U.S.A. products: Chemtrec: +1 (800) 424 9300, +1 (703) 527-3887 2. HAZARDS IDENTIFICATION 2.1 Classification of the substance or mixture Classification according to OSHA Hazards Target Organ Effect (Central Nervous System, Heart, Skeletal Muscle, Kidney), Toxic by ingestion, Toxic by skin absorption, Corrosive Rapidly absorbed through skin Classification according to GHS Classification Acute toxicity, Oral (Category 3) Acute toxicity, Inhalation (Category 3) Skin corrosion (Category 1B) Serious eye damage (Category 1) 1/12 SAFETY DATA SHEET MONOCHLOROACETIC ACID 80% - 90% AQ Solution Revision Date: September 2019 Previous date: May 2014 Print Date: September 2019 Acute aquatic toxicity (Category 1) 2.2 Label elements Labeling according to GHS Label elements, including precautionary statements Pictogram Signal word Danger Hazard statement(s) H301 + H311 Toxic if swallowed or in contact with skin H314 Causes severe skin burns and eye damage. -

Chloroacetic Acid ≥99,5 %, P.A
Safety data sheet according to Regulation (EC) No. 1907/2006 (REACH) Chloroacetic acid ≥99,5 %, p.a. article number: 9849 date of compilation: 2016-06-17 Version: 1.1 en Revision: 2021-02-17 Replaces version of: 2016-06-17 Version: (1) SECTION 1: Identification of the substance/mixture and of the company/ undertaking 1.1 Product identifier Identification of the substance Chloroacetic acid ≥99,5 %, p.a. Article number 9849 Registration number (REACH) It is not required to list the identified uses be- cause the substance is not subject to registration according to REACH (< 1 t/a). Index number in CLP Annex VI 607-003-00-1 EC number 201-178-4 CAS number 79-11-8 1.2 Relevant identified uses of the substance or mixture and uses advised against Relevant identified uses: Laboratory chemical Laboratory and analytical use Uses advised against: Do not use for squirting or spraying. Do not use for products which come into direct contact with the skin. Do not use for products which come in- to contact with foodstuffs. Do not use for private purposes (household). 1.3 Details of the supplier of the safety data sheet Carl Roth GmbH + Co KG Schoemperlenstr. 3-5 D-76185 Karlsruhe Germany Telephone:+49 (0) 721 - 56 06 0 Telefax: +49 (0) 721 - 56 06 149 e-mail: [email protected] Website: www.carlroth.de Competent person responsible for the safety data :Department Health, Safety and Environment sheet: e-mail (competent person): [email protected] 1.4 Emergency telephone number Name Street Postal Telephone Website code/city National Poisons Information Beaumont Road Dublin 9 01 809 2166 https:// Centre www.poisons.ie/ Beaumont Hospital Ireland (en) Page 1 / 17 Safety data sheet according to Regulation (EC) No. -

United States Patent (19) (11) 4,129,595 Suzuki 45) Dec
United States Patent (19) (11) 4,129,595 Suzuki 45) Dec. 12, 1978 (54) PREPARATION OF CHLOROACETYL (56) References Cited CHLORDE PUBLICATIONS E. E. Blaise et al., Comptes Rendus (France), vol. 174, 75 Inventor: Shigeto Suzuki, San Francisco, Calif. pp. 1173-1174, (1922), (Chem. Abstr., vol. 16, 2480). 73) Assignee: Chevron Research Company, San Primary Examiner-Gerald A. Schwartz Francisco, Calif. Attorney, Agent, or Firm-D. A. Newell; John Stoner, Jr. (21) Appl. No.: 891,429 57 ABSTRACT Chloroacetyl chloride is prepared by reacting glycolic (22) Filed: Mar. 29, 1978 acid with thionyl chloride in the presence of nitrogen 51) int. C.’.............................................. CO7C 51/58 containing organic compound orphosphine compound. 52 U.S. C. ................................................ 260/544 Y 58 Field of Search .................................... 260/544 Y 3 Claims, No Drawings 4,129,595 1. 2 glycolic acid with thionyl chloride in the presence of a PREPARATION OF CHLOROACETYL CHLORDE catalytic amount of nitrogen-containing hydrocarbyl organic compound or hydrocarbyl phosphine com BACKGROUND OF THE INVENTION pound at high conversion and yield in accordance with 1. Field of the Invention 5 the present invention. The present invention relates to the preparation of chloroacetyl chloride. More particularly, the invention DETALED DESCRIPTION OF THE relates to the preparation of chloroacetyl chloride by INVENTION reacting glycolic acid with thionyl chloride in the pres The nitrogen-containing hydrocarbyl organic com ence of nitrogen-containing -

Ketene Reactions. I. the Addition of Acid Chlorides
KETENE REACTIONS. I. THE ADDITION OF ACID CHLORIDES TO DIMETHYLKETENE. II. THE CYCLOADDITION OF KETENES TO CARBONYL COMPOUNDS APPROVED: Graduate Committee: Major Professor Committee Member.rr^- Committee Member Committee Member Director of the Department of Chemistry Dean' of the Graduate School Smith, Larry, Ketene Reactions. I. The Addition of Acid Chlorides to DimethyIketene. II. The Cycloaddition of Ketenes to Carbonvl Compounds. Doctor of Philosophy (Chemistry), December, 1970, 63 pp., 3 tables, bibliography, 62 titles. Part I describes the addition of several acid chlorides to dimethylketene. The resulting 3-ketoacid chlorides were isolated and characterized. The reactivities of acid chlorides were found to parallel the parent acid pKa's. A reactivity order of ketenes toward acid chlorides was established. Dimethylketene is more reactive than ketene which is more reactive than diphenylketene. Attempts to effect the addition of an acid halide to a ketene produced by in situ dehydro- halogenation yielded a-halovinyl esters. The addition of acid chlorides to ketenes was concluded to be an ionic process dependent upon the nucleophilic character of the ketene oc- carbon and the polarity of the carbon-chlorine bond in the acid chloride. Part II describes the cycloaddition of several aldo- ketenes to chloral. The ketenes were generated in situ by dehydrohalogenation and dehalogenation of appropriately substituted acyl halides. Both cis- and trans-4-trichloro- Miyl-2-oxetanones are produced in the cycloadditions with the sterically hindered cis isomer predominating. Isomer distributions were determined by vpc or nmr analysis of the reaction solutions. Production of the ketenes by dehalo- genation resulted in enhanced reactivity of the carbonyl compounds. -

Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid
Asian Journal of Chemistry; Vol. 26, No. 2 (2014), 475-480 http://dx.doi.org/10.14233/ajchem.2014.15484 Study on Gas-Phase Mechanism of Chloroacetic Acid Synthesis by Catalysis and Chlorination of Acetic Acid * JIAN-WEI XUE , JIAN-PENG ZHANG, BO WU, FU-XIANG LI and ZHI-PING LV Research Institute of Special Chemicals, Taiyuan University of Technology, Taiyuan 030024, Shanxi Province, P.R. China *Corresponding author: Fax: +86 351 6111178; Tel: +86 351 60105503; E-mail: [email protected] Received: 14 March 2013; Accepted: 17 May 2013; Published online: 15 January 2014; AJC-14570 The process of acetic acid catalysis and chlorination for synthesizing chloroacetic acid can exist in not only gas phase but also liquid phase. In this paper, the gas-phase reaction mechanism of the synthesis of chloroacetic acid was studied. Due to the high concentration of acetic acid and the better reaction mass transfer in the liquid-phase reaction, the generation amount of the dichloroacetic acid was higher than that in the gas-phase reaction. Under the solution distillation, the concentration of acetyl chloride, whose boiling point is very low, was very high in the gas phase, sometimes even up to 99 %, which would cause the acetyl chloride to escape rapidly with the hydrogen chloride exhaust, so that the reaction slowed down. Therefore, series reactions occured easily in the gas-phase reaction causing the amount of the dichloroacetic acid to increase. Keywords: Gas phase, Catalysis, Chlorination, Chloroacetic acid, Acetic acid. INTRODUCTION Martikainen et al.3 summed up the reaction mechanism that was consistent with a mechanism found by Sioli according Chloroacetic acid is not only a fine chemical product but to the system condition experiment and systematic theoretical also an important intermediate in organic synthesis.