Synthesis and Evaluation of Novel Biologically Active Compounds
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Acidity, Basicity, and Pka 8 Connections
Acidity, basicity, and pKa 8 Connections Building on: Arriving at: Looking forward to: • Conjugation and molecular stability • Why some molecules are acidic and • Acid and base catalysis in carbonyl ch7 others basic reactions ch12 & ch14 • Curly arrows represent delocalization • Why some acids are strong and others • The role of catalysts in organic and mechanisms ch5 weak mechanisms ch13 • How orbitals overlap to form • Why some bases are strong and others • Making reactions selective using conjugated systems ch4 weak acids and bases ch24 • Estimating acidity and basicity using pH and pKa • Structure and equilibria in proton- transfer reactions • Which protons in more complex molecules are more acidic • Which lone pairs in more complex molecules are more basic • Quantitative acid/base ideas affecting reactions and solubility • Effects of quantitative acid/base ideas on medicine design Note from the authors to all readers This chapter contains physical data and mathematical material that some readers may find daunting. Organic chemistry students come from many different backgrounds since organic chemistry occu- pies a middle ground between the physical and the biological sciences. We hope that those from a more physical background will enjoy the material as it is. If you are one of those, you should work your way through the entire chapter. If you come from a more biological background, especially if you have done little maths at school, you may lose the essence of the chapter in a struggle to under- stand the equations. We have therefore picked out the more mathematical parts in boxes and you should abandon these parts if you find them too alien. -

Preparation and Purification of Atmospherically Relevant Α
Atmos. Chem. Phys., 20, 4241–4254, 2020 https://doi.org/10.5194/acp-20-4241-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Technical note: Preparation and purification of atmospherically relevant α-hydroxynitrate esters of monoterpenes Elena Ali McKnight, Nicole P. Kretekos, Demi Owusu, and Rebecca Lyn LaLonde Chemistry Department, Reed College, Portland, OR 97202, USA Correspondence: Rebecca Lyn LaLonde ([email protected]) Received: 31 July 2019 – Discussion started: 6 August 2019 Revised: 8 December 2019 – Accepted: 20 January 2020 – Published: 9 April 2020 Abstract. Organic nitrate esters are key products of terpene oxidation in the atmosphere. We report here the preparation and purification of nine nitrate esters derived from (C)-3- carene, limonene, α-pinene, β-pinene and perillic alcohol. The availability of these compounds will enable detailed investigations into the structure–reactivity relationships of aerosol formation and processing and will allow individual investigations into aqueous-phase reactions of organic nitrate esters. Figure 1. Two hydroxynitrate esters with available spectral data. Relative stereochemistry is undefined. 1 Introduction derived ON is difficult, particularly due to partitioning into the aerosol phase in which hydrolysis and other reactivity Biogenic volatile organic compound (BVOC) emissions ac- can occur (Bleier and Elrod, 2013; Rindelaub et al., 2014, count for ∼ 88 % of non-methane VOC emissions. Of the to- 2015; Romonosky et al., 2015; Thomas et al., 2016). Hydrol- tal BVOC estimated by the Model of Emission of Gases and ysis reactions of nitrate esters of isoprene have been stud- Aerosols from Nature version 2.1 (MEGAN2.1), isoprene is ied directly (Jacobs et al., 2014) and the hydrolysis of ON estimated to comprise half, and methanol, ethanol, acetalde- has been studied in bulk (Baker and Easty, 1950). -

Thermal Decomposition of Nitrate Esters1 Michael A. Hiskey, Kay R. Brower, and Jimmie C. Oxley* Department of Chemistry New Mexi
Thermal Decomposition of Nitrate Esters1 Michael A. Hiskey, Kay R. Brower, and Jimmie C. Oxley* Department of Chemistry New Mexico Institute of Mining and Technology Socorro, New Mexico 87801 Abstract Rates of thermal decomposition and solvent rate effects have been measured for a series of nitrate esters. The alkoxy radicals formed by homolysis together with some of their further degradation products have been stabilized by hydrogen donation. Internal and external return of nitrogen dioxide has been demonstrated by solvent cage effects and isotope exchange. Radical- stabilizing substituents favor β-scission. Dinitrates in a 1,5 relationship behave as isolated mononitrates. Dinitrates in a 1,3 or 1,4 relationship exhibit intramolecular reactions. Tertiary nitrate esters in diethyl ether undergo elimination rather than homolysis. Introduction The esters of nitric acid have long been used as explosives and propellants, and their thermal decomposition products and kinetics have been studied as a means to evaluate the thermal hazards of these compounds. The thermal decomposition of ethanolnitrate was examined by a number of researchers, and it was proposed that the first, and rate-determining, step was the 2-5 reversible loss of NO2: . RCH2O-NO2 <--> RCH2O + NO2 Griffiths, Gilligan, and Gray6 investigated 2-propanol nitrate pyrolysis and found it exhibited more β-cleavage than primary nitrate esters. It yielded nearly equal amounts of 2- propanol nitrite and acetaldehyde, as well as small amounts of acetone, nitromethane, and methyl nitrite (Fig. 1). Homolytic cleavage of the RO-NO2 bond would form the 2-propoxy radical, which could subsequently react with nitric oxide to produce 2-propanol nitrite. -
1 the 2-Step Synthesis of Lidocaine Review
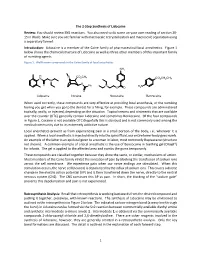
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

Organic Functional Group Analysis
Experiment 1 1 Laboratory Experiments for GOB Chemistry ___________________________________________________________________________________________ I ORGANIC FUNCTIONAL GROUP ANALYSIS I. OBJECTIVES AND BACKGROUND This experiment will introduce you to some of the more common functional groups of organic chemistry. The functional group is that portion of the molecule that undergoes a structural change during a chemical reaction. The functional groups that will be studied in this experiment are carboxylic acid, amines aldehyde, ketone, alcohols and alkenes. You will learn chemical tests that will allow you to distinguish one functional group from another. You will use the chemical tests to identify the functionality of an unknown organic compound. In addition, you will use a water solubility test to determine whether your organic compound is of high or low formula weight. The chemical tests you will perform make up a sequence of experiments designed to determine the absence of or suggest the presence of particular functional groups. The complete sequence is shown in the flow diagram on page 8. This diagram can serve you in several ways: It is a summary of the procedure that you are to follow in classifying your unknown as one of the functional group types. It can order your thoughts as you read the discussion of each test, and help you to understand the significance of that test. It can enhance your appreciation for and enjoyment of this experiment. Your role is that of chemist and detective: you will employ this cleverly devised scheme to sleuth out the identity of your unknown's functionality. Experiment 1 2 Laboratory Experiments for GOB Chemistry ___________________________________________________________________________________________ Discussion of Chemical Tests 1. -

United States Patent (19) 11 Patent Number: 5,807,847 Thatcher Et Al
USOO5807847A United States Patent (19) 11 Patent Number: 5,807,847 Thatcher et al. (45) Date of Patent: Sep. 15, 1998 54 NITRATE ESTERS Bennett, B.M., McDonald, B.J., Nigam, R., and Simon, W.C., “Biotransformation of organic nitrates and vascular 75 Inventors: Gregory R. J. Thatcher; Brian M. Smooth muscle cell function', Trends in Pharmacol. Sci. 15: Bennett, both of Kingston, Canada 245–249 (1994). Cameron, D.R., Borrajo, A.M.P., Bennett, B.M., and 73 Assignee: Queen's University at Kingston, Thatcher, G.R.J., “Organic nitrates, thionitrates, peroxyni Kingston, Canada trites, and nitric oxide: a molecular orbital study of RXNO es RXONO (X=O.S) rearrangement, a reaction of potential 21 Appl. No.: 658,145 biological significance”, Can. J. Chem. 73: 1627-1638 22 Filed: Jun. 4, 1996 (1995). Chong, S., and Fung, H.-L., “Biochemical and pharmaco 51) Int. Cl. ...................... C07C 38/102; CO7C 203/04; logical interactions between nitroglycerin and thiols. Effects A61K 31/255; A61K 31/66; A61K 31/39; of thiol Structure on nitric oxide generation and tolerance A61K 31/21; CO7F 9/40; CO7D 327/04 reversal”, Biochem. Pharm. 42: 1433–1439 (1991). 52 U.S. Cl. .............................. 514/129; 514/23: 514/24; 514/439; 514/517; 514/509; 536/18.7; 536/55; Feelisch, M., “Biotransformation to nitric oxide of organic 549/40; 558/175; 558/480; 558/484; 558/485; nitrates in comparison to other nitrovasodilators”, Eur: Heart 560/309 J., 14: 123–132 (1993). 58 Field of Search ..................................... 514/129, 439, Fung, H-L., "Nitrate therapy: is there an optimal Substance 514/509, 517; 549/40; 558/175, 480, 484, and formulation'Eur: Heart J., 12:9-12 (1991). -

Comparative Strengths of Four Organic Bases in Benzene1 Marion Maclean Davis and Hannah B
Journal of Research of the National Bureau of Standards Vol. 48, No. 5, May 1952 Research Paper 2326 Comparative Strengths of Four Organic Bases in Benzene1 Marion Maclean Davis and Hannah B. Hetzer Spectropho to metric studies have shown that the reaction of the base 1,3-di-o-tolylguani- dine with the acidic indicator dye bromophthalein magenta E (tetrabromophenolphthalein ethyl ester) in benzene at 25° C, like the reactions of 1,3-diphenylguanidine and 1,2,3-tri- phenylguanidine with the same indicator, can be represented by the following two equations: B + HA ^ BH+.A- (colorless base) (yellow acid) (magenta salt) BH+.A- + B^(BHB)+A- (blue salt) For ditolylguanidine, the equilibrium constants K\ and K2 for the first and second reactions, respectively, are estimated to be 1.1 X106 and 6.4. These values are compared with values for Ki and K2 previously found for di- and triphenylguanidine and the value of Ki found for triethylamine. The values for K\, which measure the relative tendencies of the bases to form salts with the indicator acid in benzene, would be expected to parallel the ionic dissociation constants of the bases in water. However, the parallelism is not good. Diphenylguanidine and ditolylguariidine, which are presumed to be weaker bases in water than triethylamine, are much more reactive in benzene. The results demonstrate how misleading the aqueous dissociation constants may be as a gage of the relative reactivities of bases in a nonaqueous solvent such as benzene. Steric and solvation effects are discussed. 1. Introduction i optical instruments then available, it was possible to make only roughly quantitative comparisons of Hantzsch and his coworkers were the first to J acidic strengths. -

1. A. the First Reaction Is a Friedel-Crafts Acylation (FCA), Where the Major Product Is the Para- Isomer (60% Isolated Yield)
1. a. The first reaction is a Friedel-Crafts Acylation (FCA), where the major product is the para- isomer (60% isolated yield). The second reaction is a nitration, where the incoming electrophile (nitronium ion) is directed to the ortho position of the methoxy group. The last reaction is a Wolff-Kishner reduction that converts the acetyl group into an ethyl group. The nitro group does not react under these conditions. OCH OCH OCH OCH3 3 3 3 NO2 NO2 N2H4/KOH CH3COCl/AlCl3 H2SO4/HNO3 0 oC O CH 3 O CH3 CH3 (A) Reaction 1 (B) Reaction 2 (C) Reaction 3 (P) b. The best solvent for the FC-acylation is dichloromethane. Tetrahydrofuran is a fairly strong Lewis base, which would react and deactivate the AlCl3 catalyst. Ethanol would also react with AlCl3 and form alcoholates, which are inactive at FCA catalyst. Dichloromethane is polar enough to dissolve all three compounds but does not form adducts with AlCl3. Thus, aluminum chloride maintains its Lewis acidity. 3+ c. As discussed in lecture, AlCl3*6 H2O is not suitable as catalyst because the Al is not a strong Lewis acid anymore. In addition, larger amounts of water would destroy the acetyl chloride as well (=hydrolysis, CH3COCl + H2O ---- > CH3COOH + HCl). Consequently, the reaction would not proceed in the desired fashion. 3+ OH2 H2O OH2 Al H2O OH2 OH2 d. In order to determine the yield, one has to calculate the number of moles of the reactant and the product. nA = 1.90 mL * 0.996 g/mL/108.14 g/mol = 17.5 mmol nCH3COCl = 2.49 mL * 1.104 g/mL/78.5 g/mol = 35.0 mmol nAlCl3 = 4.67 g/133.5 g/mol = 35.0 mmol Compound (A) is the limiting reagent. -

NITROGYLCERIN and ETHYLENE GLYCOL DINITRATE Criteria for a Recommended Standard OCCUPATIONAL EXPOSURE to NITROGLYCERIN and ETHYLENE GLYCOL DINITRATE
CRITERIA FOR A RECOMMENDED STANDARD OCCUPATIONAL EXPOSURE TO NITROGYLCERIN and ETHYLENE GLYCOL DINITRATE criteria for a recommended standard OCCUPATIONAL EXPOSURE TO NITROGLYCERIN and ETHYLENE GLYCOL DINITRATE U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health June 1978 For »ale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DISCLAIMER Mention of company name or products does not constitute endorsement by the National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. 78-167 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and provide for the safety of workers occupationally exposed to an ever-increasing number of potential hazards. The National Institute for Occupational Safety and Health (NIOSH) evaluates all available research data and criteria and recommends standards for occupational exposure. The Secretary of Labor will weigh these recommendations along with other considerations, such as feasibility and means of implementation, in promulgating regulatory standards. NIOSH will periodically review the recommended standards to ensure continuing protection of workers and will make successive reports as new research and epidemiologic studies are completed and as sampling and analytical methods are developed. The contributions to this document on nitroglycerin (NG) and ethylene glycol dinitrate (EGDN) by NIOSH staff, other Federal agencies or departments, the review consultants, the reviewers selected by the American Industrial Hygiene Association, and by Robert B. O ’Connor, M.D., NIOSH consultant in occupational medicine, are gratefully acknowledged. The views and conclusions expressed in this document, together with the recommendations for a standard, are those of NIOSH. -

United States Patent (19) (11) 4,129,595 Suzuki 45) Dec
United States Patent (19) (11) 4,129,595 Suzuki 45) Dec. 12, 1978 (54) PREPARATION OF CHLOROACETYL (56) References Cited CHLORDE PUBLICATIONS E. E. Blaise et al., Comptes Rendus (France), vol. 174, 75 Inventor: Shigeto Suzuki, San Francisco, Calif. pp. 1173-1174, (1922), (Chem. Abstr., vol. 16, 2480). 73) Assignee: Chevron Research Company, San Primary Examiner-Gerald A. Schwartz Francisco, Calif. Attorney, Agent, or Firm-D. A. Newell; John Stoner, Jr. (21) Appl. No.: 891,429 57 ABSTRACT Chloroacetyl chloride is prepared by reacting glycolic (22) Filed: Mar. 29, 1978 acid with thionyl chloride in the presence of nitrogen 51) int. C.’.............................................. CO7C 51/58 containing organic compound orphosphine compound. 52 U.S. C. ................................................ 260/544 Y 58 Field of Search .................................... 260/544 Y 3 Claims, No Drawings 4,129,595 1. 2 glycolic acid with thionyl chloride in the presence of a PREPARATION OF CHLOROACETYL CHLORDE catalytic amount of nitrogen-containing hydrocarbyl organic compound or hydrocarbyl phosphine com BACKGROUND OF THE INVENTION pound at high conversion and yield in accordance with 1. Field of the Invention 5 the present invention. The present invention relates to the preparation of chloroacetyl chloride. More particularly, the invention DETALED DESCRIPTION OF THE relates to the preparation of chloroacetyl chloride by INVENTION reacting glycolic acid with thionyl chloride in the pres The nitrogen-containing hydrocarbyl organic com ence of nitrogen-containing