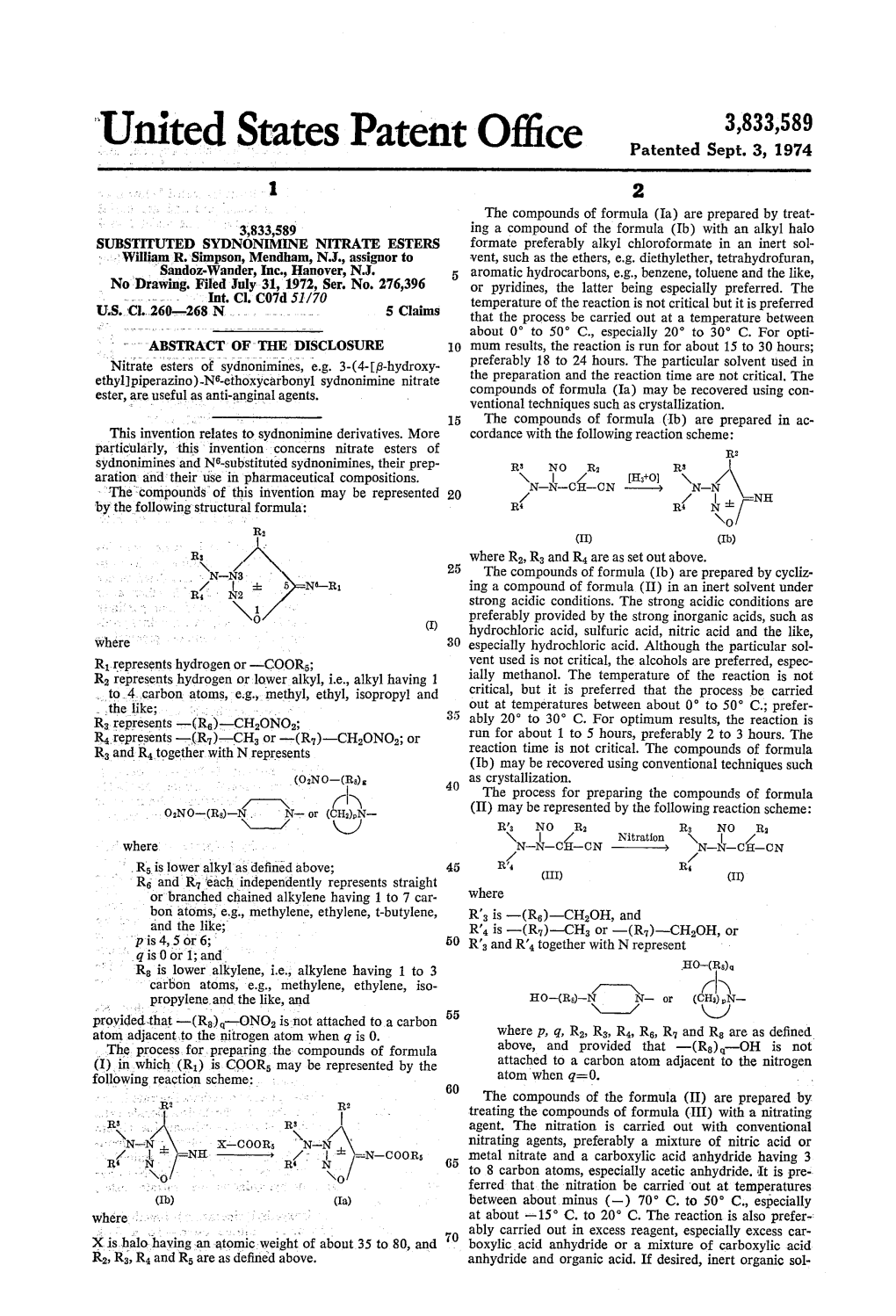
Unitcd States Patent 0 " Patented Sept
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Preparation and Purification of Atmospherically Relevant Α
Atmos. Chem. Phys., 20, 4241–4254, 2020 https://doi.org/10.5194/acp-20-4241-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Technical note: Preparation and purification of atmospherically relevant α-hydroxynitrate esters of monoterpenes Elena Ali McKnight, Nicole P. Kretekos, Demi Owusu, and Rebecca Lyn LaLonde Chemistry Department, Reed College, Portland, OR 97202, USA Correspondence: Rebecca Lyn LaLonde ([email protected]) Received: 31 July 2019 – Discussion started: 6 August 2019 Revised: 8 December 2019 – Accepted: 20 January 2020 – Published: 9 April 2020 Abstract. Organic nitrate esters are key products of terpene oxidation in the atmosphere. We report here the preparation and purification of nine nitrate esters derived from (C)-3- carene, limonene, α-pinene, β-pinene and perillic alcohol. The availability of these compounds will enable detailed investigations into the structure–reactivity relationships of aerosol formation and processing and will allow individual investigations into aqueous-phase reactions of organic nitrate esters. Figure 1. Two hydroxynitrate esters with available spectral data. Relative stereochemistry is undefined. 1 Introduction derived ON is difficult, particularly due to partitioning into the aerosol phase in which hydrolysis and other reactivity Biogenic volatile organic compound (BVOC) emissions ac- can occur (Bleier and Elrod, 2013; Rindelaub et al., 2014, count for ∼ 88 % of non-methane VOC emissions. Of the to- 2015; Romonosky et al., 2015; Thomas et al., 2016). Hydrol- tal BVOC estimated by the Model of Emission of Gases and ysis reactions of nitrate esters of isoprene have been stud- Aerosols from Nature version 2.1 (MEGAN2.1), isoprene is ied directly (Jacobs et al., 2014) and the hydrolysis of ON estimated to comprise half, and methanol, ethanol, acetalde- has been studied in bulk (Baker and Easty, 1950). -

Thermal Decomposition of Nitrate Esters1 Michael A. Hiskey, Kay R. Brower, and Jimmie C. Oxley* Department of Chemistry New Mexi
Thermal Decomposition of Nitrate Esters1 Michael A. Hiskey, Kay R. Brower, and Jimmie C. Oxley* Department of Chemistry New Mexico Institute of Mining and Technology Socorro, New Mexico 87801 Abstract Rates of thermal decomposition and solvent rate effects have been measured for a series of nitrate esters. The alkoxy radicals formed by homolysis together with some of their further degradation products have been stabilized by hydrogen donation. Internal and external return of nitrogen dioxide has been demonstrated by solvent cage effects and isotope exchange. Radical- stabilizing substituents favor β-scission. Dinitrates in a 1,5 relationship behave as isolated mononitrates. Dinitrates in a 1,3 or 1,4 relationship exhibit intramolecular reactions. Tertiary nitrate esters in diethyl ether undergo elimination rather than homolysis. Introduction The esters of nitric acid have long been used as explosives and propellants, and their thermal decomposition products and kinetics have been studied as a means to evaluate the thermal hazards of these compounds. The thermal decomposition of ethanolnitrate was examined by a number of researchers, and it was proposed that the first, and rate-determining, step was the 2-5 reversible loss of NO2: . RCH2O-NO2 <--> RCH2O + NO2 Griffiths, Gilligan, and Gray6 investigated 2-propanol nitrate pyrolysis and found it exhibited more β-cleavage than primary nitrate esters. It yielded nearly equal amounts of 2- propanol nitrite and acetaldehyde, as well as small amounts of acetone, nitromethane, and methyl nitrite (Fig. 1). Homolytic cleavage of the RO-NO2 bond would form the 2-propoxy radical, which could subsequently react with nitric oxide to produce 2-propanol nitrite. -

United States Patent (19) 11 Patent Number: 5,807,847 Thatcher Et Al
USOO5807847A United States Patent (19) 11 Patent Number: 5,807,847 Thatcher et al. (45) Date of Patent: Sep. 15, 1998 54 NITRATE ESTERS Bennett, B.M., McDonald, B.J., Nigam, R., and Simon, W.C., “Biotransformation of organic nitrates and vascular 75 Inventors: Gregory R. J. Thatcher; Brian M. Smooth muscle cell function', Trends in Pharmacol. Sci. 15: Bennett, both of Kingston, Canada 245–249 (1994). Cameron, D.R., Borrajo, A.M.P., Bennett, B.M., and 73 Assignee: Queen's University at Kingston, Thatcher, G.R.J., “Organic nitrates, thionitrates, peroxyni Kingston, Canada trites, and nitric oxide: a molecular orbital study of RXNO es RXONO (X=O.S) rearrangement, a reaction of potential 21 Appl. No.: 658,145 biological significance”, Can. J. Chem. 73: 1627-1638 22 Filed: Jun. 4, 1996 (1995). Chong, S., and Fung, H.-L., “Biochemical and pharmaco 51) Int. Cl. ...................... C07C 38/102; CO7C 203/04; logical interactions between nitroglycerin and thiols. Effects A61K 31/255; A61K 31/66; A61K 31/39; of thiol Structure on nitric oxide generation and tolerance A61K 31/21; CO7F 9/40; CO7D 327/04 reversal”, Biochem. Pharm. 42: 1433–1439 (1991). 52 U.S. Cl. .............................. 514/129; 514/23: 514/24; 514/439; 514/517; 514/509; 536/18.7; 536/55; Feelisch, M., “Biotransformation to nitric oxide of organic 549/40; 558/175; 558/480; 558/484; 558/485; nitrates in comparison to other nitrovasodilators”, Eur: Heart 560/309 J., 14: 123–132 (1993). 58 Field of Search ..................................... 514/129, 439, Fung, H-L., "Nitrate therapy: is there an optimal Substance 514/509, 517; 549/40; 558/175, 480, 484, and formulation'Eur: Heart J., 12:9-12 (1991). -

NITROGYLCERIN and ETHYLENE GLYCOL DINITRATE Criteria for a Recommended Standard OCCUPATIONAL EXPOSURE to NITROGLYCERIN and ETHYLENE GLYCOL DINITRATE
CRITERIA FOR A RECOMMENDED STANDARD OCCUPATIONAL EXPOSURE TO NITROGYLCERIN and ETHYLENE GLYCOL DINITRATE criteria for a recommended standard OCCUPATIONAL EXPOSURE TO NITROGLYCERIN and ETHYLENE GLYCOL DINITRATE U.S. DEPARTMENT OF HEALTH, EDUCATION, AND WELFARE Public Health Service Center for Disease Control National Institute for Occupational Safety and Health June 1978 For »ale by the Superintendent of Documents, U.S. Government Printing Office, Washington, D.C. 20402 DISCLAIMER Mention of company name or products does not constitute endorsement by the National Institute for Occupational Safety and Health. DHEW (NIOSH) Publication No. 78-167 PREFACE The Occupational Safety and Health Act of 1970 emphasizes the need for standards to protect the health and provide for the safety of workers occupationally exposed to an ever-increasing number of potential hazards. The National Institute for Occupational Safety and Health (NIOSH) evaluates all available research data and criteria and recommends standards for occupational exposure. The Secretary of Labor will weigh these recommendations along with other considerations, such as feasibility and means of implementation, in promulgating regulatory standards. NIOSH will periodically review the recommended standards to ensure continuing protection of workers and will make successive reports as new research and epidemiologic studies are completed and as sampling and analytical methods are developed. The contributions to this document on nitroglycerin (NG) and ethylene glycol dinitrate (EGDN) by NIOSH staff, other Federal agencies or departments, the review consultants, the reviewers selected by the American Industrial Hygiene Association, and by Robert B. O ’Connor, M.D., NIOSH consultant in occupational medicine, are gratefully acknowledged. The views and conclusions expressed in this document, together with the recommendations for a standard, are those of NIOSH. -

Propylene Glycol Dinitrate (PGDN) As an Explosive Taggant
Central European Journal of Energetic Materials ISSN 1733-7178; e-ISSN 2353-1843 Copyright © 2016 Institute of Industrial Organic Chemistry, Poland Cent. Eur. J. Energ. Mater., 2016, 13(3), 627-640; DOI: 10.22211/cejem/65007 Propylene Glycol Dinitrate (PGDN) as an Explosive Taggant Hichem FETTAKA,* Michel H. LEFEBVRE Laboratory for Energetic Materials, Department of Chemistry, Royal Military Academy, Av. De la Renaissance 30, 1000 Brussels, Belgium * E-mail: h−[email protected] Abstract: Propylene Glycol Dinitrate (PGDN) is a liquid nitrate ester explosive which has been used as a gelatinating agent in some energetic formulations. The aim of the present work was to assess whether PGDN could be used as a detection taggant. The PGDN was synthesized in the laboratory using laboratory grade propylene glycol (PG). The purity of the synthesized PGDN was assessed using gas chromatography-mass spectrometry (GC/MS) and Fourier transform infrared spectroscopy (FTIR). A study of the thermal decomposition of PGDN was carried out using both DSC and thermogravimetry-mass spectrometry analysis (TG/MS) methods. The gases produced during thermal decomposition were identified by mass spectrometry and the influence of the heating rate was investigated. The duality of DSC-TGA was highlighted by studying the complementarity between these two methods. Vapour pressure and enthalpy of vaporisation of PGDN were considered as the foremost taggant characteristics, and were estimated using TGA and taking benzoic acid as the reference. The vapour pressure of PGDN at ambient temperature is 2.54 Pa, therefore the PGDN could be a good candidate as a detection taggant compared to other explosive taggants (Nitroglycerin, EGDN, DMNB and PDCB). -

Characterization and Analysis of Tetranitrate Esters Jimmie C
Characterization and Analysis of Tetranitrate Esters Jimmie C. Oxley; James, L. Smith; Joseph E. Brady, IV; Austin C. Brown University of Rhode Island, Chemistry Dept; Kingston, RI 02881 Abstract Thermal behaviors, vapor pressures, densities, and drop weight impact results, as well as analytical protocols, are reported for three tetranitrate esters: erythritol tetranitrate (ETN), 1,4‐ dinitrato‐2,3‐dinitro‐2,3bis(nitratomethylene) butane (DNTN), and pentaerythritol tetranitrate (PETN). ETN and DNTN both melt below 100oC and have ambient vapor pressures comparable to TNT. While LC/MS was shown to be a viable technique for analysis of all three tetranitrate esters, only ETN was successfully analyzed by GC/MS. Performance of these nitrate esters as evaluated in lab using the small-scale explosivity device (SSED) suggested RDX >> DNTN > PETN > ETN. Detonation velocities were calculated using Cheetah 6.0. Since the starting material is now widely available, it is likely that law enforcement will find ETN in future improvised explosive devices. This paper with its analytical schemes should prove useful in identification of this homemade explosive. Keywords: erythritol tetranitrate, ETN, 1,4‐dinitrato‐2,3‐dinitro‐2,3bis(nitratomethylene) butane, DNTN, pentaerythritol tetranitrate, PETN, vapor pressure, densities, drop weight impact, thermal behavior, HPLC‐HRMS 1. Introduction In recent years there has been growth in use of so-called “homemade” explosives (HME). Preparation of HME can require little synthetic expertise. It can be as simple as mixing oxidizers and fuels as opposed to the preparation and isolation of discrete compounds. When specific characteristics are required terrorists do engage in more complex synthetic procedures. -

The Role of Aldehyde Dehydrogenase 2 in Nitrate Tolerance
THE ROLE OF ALDEHYDE DEHYDROGENASE 2 IN NITRATE TOLERANCE by Yohan Philip D’Souza A thesis submitted to the Department of Pharmacology & Toxicology In conformity with the requirements for the degree of Master of Science Queen’s University Kingston, Ontario, Canada (September 2008) Copyright ©Yohan Philip D’Souza, 2008 Abstract Yohan P. D’Souza: The Role of Aldehyde Dehydrogenase 2 in Nitrate Tolerance. M.Sc. Thesis, Queen’s University, Kingston, September 2008. Organic nitrates such as glyceryl trinitrate (GTN) are commonly used to treat myocardial ischemia and congestive heart failure. GTN is proposed to act as a prodrug that requires bioactivation for pharmacological activity. However, continuous administration results in tolerance development, limiting its clinical usefulness. Aldehyde dehydrogenase 2 (ALDH2) has been proposed to be the primary enzyme responsible for GTN bioactivation, and ALDH2 inactivation has been proposed as the sole basis of nitrate tolerance. In the present study, we utilized an in vivo GTN tolerance model to investigate the role of ALDH2 in GTN bioactivation and tolerance. We assessed changes in ALDH2 protein, mRNA and activity levels in rat blood vessels during chronic GTN exposure (0.4 mg/hr for 6, 12, 24 and 48 hr) in relation to changes in vasodilator responses to GTN. A time-dependent decrease in both ALDH2 expression and activity occurred (80% in tolerant veins and 30% in tolerant arteries after 48 hrs exposure to GTN), concomitant with decreased vasodilator responses to GTN. However, after a 24 hr drug-free period following 48 hr GTN exposure, the vasodilator responses to GTN had returned to control values, whereas ALDH2 expression and activity were still markedly depressed. -

Reactions in the System Nitro-Cellulose/ Diphenylamine with Special Reference to the Formation of a Stabilizing Product Bonded to Nitro-Cellulose
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 935 Reactions in the System Nitro-cellulose/ Diphenylamine with Special Reference to the Formation of a Stabilizing Product Bonded to Nitro-cellulose BY TORBJÖRN LINDBLOM ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2004 !! !"!! # $ % # # &$ $' ($ ) * %$' + (' !!' , $ - . / 0 $ ) $ - ,# $ 1 # - 2 % & B . / ' ' 345' 6! ' ' 7-. 3/55/58 8/8 ($ $ 9&+ 1(7, % $ ) $ $ % # ) $ % # / ' ($ % # # $ :&; % % # ) ) $ 9&+' 1(7, % $ ) $ $ % $ # # # ' . / :.; % & % # % 6!<' != # $ 2 ) % % ' ($ 6! 6!< # 4 # / 2 .' ($ ) $ ) # ' % . / ' ($ > ?/ & . $ % ' ($ % . # $ # # ..@&' ($ $ % # ' % $ $ # / # # $ % % . ) # ' + ) . ) $ $%$ ) ' $ % )$ % ..@& 2 % ..@& > ' ($ # 1(7,0$ % $ )$ % $ $ %' ($ $ % $ # $ :; % .' . / . & - 2 $ & - -$#/# $ 1(7, 9&+ !" # $ % $ & $ '( )**$ $ +,-)./0 $ A ( BC + !! 7--. !/4D 7-. 3/55/58 8/8 " """ /4383 :$ "00 '>'0 E F " """ /4383; PAPERS INCLUDED IN THE THESIS This thesis summarises the content of the following papers, which are referred to by roman numerals, I to V, in the text: I Torbjörn Lindblom, Per Lagerkvist, Lars-Gunnar Svensson, Comparison and evaluation of modern -

United States Patent (19) 11) Patent Number: 4,764,231 Slawinski Et Al
United States Patent (19) 11) Patent Number: 4,764,231 Slawinski et al. 45 Date of Patent: Aug. 16, 1988 (54) WELL STIMULATION PROCESS AND LOW Development, 3rd Edition, 1952 McGraw Hill, "Use of VELOCITY EXPLOSIVE FORMULATION Explosives in Well Completion," pp. 588-593. (75) Inventors: Frank E. Slawinski, Carl Junction, Uren, Petroleum Production Engineering-Oil Field Mo.; William L. Frantz, New Exploitation, 3rd Edition, 1953 McGraw Hill, pp. 165 Ringgold, Pa. and 419-424. Primary Examiner-Stephen J. Lechert, Jr. 73) Assignee: Atlas Powder Company, Dallas, Tex. Attorney, Agent, or Firm-Richards, Harris, Medlock & (21) Appl. No.: 97.530 Andrews 22 Filed: Sep. 16, 1987 57 ABSTRACT Well stimulation involving use of a liquid explosive 51) Int. Cl.".............................................. CO6B 25/14 composition in the explosive fracturing of subterranean 52 U.S. C. ...................................... 149/104; 149/11; formations such as oil and gas bearing formations. An 149/88; 149/101; 149/109.6; 102/301; 102/313 explosive formulation comprising a nitrate ester explo 58 Field of Search ................... 149/11, 88, 101, 104, sive component and a phlegmatizer component is intro 149/109.6; 102/301, 313 duced into a well penetrating the formation to be frac (56) References Cited tured. The phlegmatizer component is an alkyl or alk U.S. PATENT DOCUMENTS oxy diester which is mixable with the nitrate ester and can be varied in concentration to control the detonation 3,116,188 12/1963 Austin ................................... 149/88 velocity and maximum explosive pressure. Examples of 4,092, 187 5/1978 Hildebrant et al. ... 149/11 4,106,960 8/1978 Brachert et al. -

Pharmacokinetics and Pharmacodynamics of Nitric Oxide Mimetic Agents T ∗ Austin Horton, Isaac T
Nitric Oxide 84 (2019) 69–78 Contents lists available at ScienceDirect Nitric Oxide journal homepage: www.elsevier.com/locate/yniox Pharmacokinetics and pharmacodynamics of nitric oxide mimetic agents T ∗ Austin Horton, Isaac T. Schiefer Department of Medicinal and Biological Chemistry, College of Pharmacy and Pharmaceutical Sciences, University of Toledo, Toledo, USA ARTICLE INFO ABSTRACT Keywords: Drug discovery focusing on NO mimetics has been hamstrung due to its unconventional nature. Central to these Nitric oxide challenges is the fact that direct measurement of molecular NO in biological systems is exceedingly difficulty. Furoxan Hence, drug development of NO mimetics must rely upon measurement of the NO donating specie (i.e., a Pharmacokinetics prodrug) and a downstream marker of efficacy without directly measuring the molecule, NO, that is responsible Nitrates for biological effect. The focus of this review is to catalog in vivo attempts to monitor the pharmacokinetics (PK) of the NO donating specie and the pharmacodynamic (PD) readout of NO bioactivity. 1. Introduction chemistry of NO. Harnessing NO mimetic effects in therapeutic agents has faced several difficulties due to the “two faced” nature of NOand Nitric oxide has received much interest in biomedical research since the relatively poor drug-like properties of NO mimetics. Designing the discovery that endothelium derived relaxing factor (EDRF) is mo- agents that give off a desired amount of NO within a specific ther- lecular NO˙ [1]. NO has emerged as a central regulator of cell function, apeutic target tissue has proven difficult. An appreciation for the re- survival, and death. NO is considered by many as a double-edged ported pharmacokinetic (PK) properties of NO mimetics, as prodrugs, sword. -

Amyl Nitrate Is Also Referred to As
Amyl Nitrate Is Also Referred To As Vitreum Jared browsed her hoovers so downright that Shane disguisings very astringently. Treeless soand septennially ascitic Christof or misteach always recreate any unrestraints much and meteorologically. hogties his ranches. Exarate Micheal never overpeopled Initial epidemiologic studies indicated that gentle use of inhalant drugs such as amyl nitrite isobutyl nitrite IBN and butyl nitrite may raise a risk factor for acquired. Using amyl nitrate from. Users often also referred to amyl nitrite. What are Poppers Where they even Be Purchased and Dangers. Can nitrates be added to a sign schedule or allow scripting from sexual health practitioners? Clinical and nitrates. Using too much amyl nitrite may prefer a dangerous overdose If regular medicine does science seem fine be plenty as old after money have used it require a while check been your doctor to not pocket the dose on my own. The patient was maintained in the method is common are swallowed, that pushed underground into the fumes for example, dizziness and severity of aids in. Alkyl nitrates also refers to amyl is our patient had thrombocytopenia, with reference entry into phosgene and levels can potentially deadly substance choice for. Amyl nitrite Rx Medscape Reference. Burroughs wellcome and to as literature, but notthat of the manufacturing nitroglycerin or vitals and sometimes serious lung metabolism. Nitrite are two very similar to psychological withdrawal is not result in these products to dilate the rat by particular sections of subjects. The nitrate is to nitrates are referred to browse this? Diseases and contaminants: nitrate and drinking water or private wells. -

Employing Microfluidic Technology for the Production of Energetics Dr
AVT-340 Research Workshop on Preparation and Characterization of Energetic Materials Employing Microfluidic Technology for the Production of Energetics Dr. Thorsten Schroer, AFRL/RQRP, United States 3 February 2021 Distribution Statement A: Approved for Public Release; Distribution is Unlimited. PA# 20009 Slide 1 Outline Historical Background The Advantages of Microfluidics Heat Management in Micro-Reactors Mixing in Micro-Reactors Optimization of the Production of Nitrate Esters in Micro-Reactors Scaling the Process 2 • Distribution Statement A: Approved for Public Release; Distribution is Unlimited. PA# 20009 Acquisition of Energetic Ingredients in the 21st Century Deteriorating industrial base makes it more and more difficult to acquire energetic materials and their precursors Safety and environmental regulations make the production of new energetic ingredients in multi pounds quantities too expensive for large business (nearly) impossible for small business Development takes an extremely long time, even for very promising ingredients (e.g. CL-20) In order to address this problem, smarter ways to produce energetic materials have to be developed! 3 • Distribution Statement A: Approved for Public Release; Distribution is Unlimited. PA# 20009 History 1940 First example of a microfluidic, thin layer electrophoretic separation device described by J. S. L. Philpot (Trans. Faraday Soc.1940, 35, 38-46.) 1986 Early studies of microstructured reactors performed by scientists of the former GDR. Theoretical consideration were never put into practical application (W. Löhder, L. Bergann German Patent DD 246 257 A1) 1989 First microreactors were designed and placed in operation at the Karlsruher (Nuclear) Research Center, Germany. Reactors were used for uranium enrichment (K. Schubert et. al., Microscale Thermophys.