Proposed TOR File
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Retention Indices for Frequently Reported Compounds of Plant Essential Oils
Retention Indices for Frequently Reported Compounds of Plant Essential Oils V. I. Babushok,a) P. J. Linstrom, and I. G. Zenkevichb) National Institute of Standards and Technology, Gaithersburg, Maryland 20899, USA (Received 1 August 2011; accepted 27 September 2011; published online 29 November 2011) Gas chromatographic retention indices were evaluated for 505 frequently reported plant essential oil components using a large retention index database. Retention data are presented for three types of commonly used stationary phases: dimethyl silicone (nonpolar), dimethyl sili- cone with 5% phenyl groups (slightly polar), and polyethylene glycol (polar) stationary phases. The evaluations are based on the treatment of multiple measurements with the number of data records ranging from about 5 to 800 per compound. Data analysis was limited to temperature programmed conditions. The data reported include the average and median values of retention index with standard deviations and confidence intervals. VC 2011 by the U.S. Secretary of Commerce on behalf of the United States. All rights reserved. [doi:10.1063/1.3653552] Key words: essential oils; gas chromatography; Kova´ts indices; linear indices; retention indices; identification; flavor; olfaction. CONTENTS 1. Introduction The practical applications of plant essential oils are very 1. Introduction................................ 1 diverse. They are used for the production of food, drugs, per- fumes, aromatherapy, and many other applications.1–4 The 2. Retention Indices ........................... 2 need for identification of essential oil components ranges 3. Retention Data Presentation and Discussion . 2 from product quality control to basic research. The identifi- 4. Summary.................................. 45 cation of unknown compounds remains a complex problem, in spite of great progress made in analytical techniques over 5. -
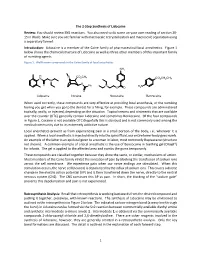
1 the 2-Step Synthesis of Lidocaine Review
The 2-Step Synthesis of Lidocaine Review: You should review SN2 reactions. You also need to do some on your own reading of section 20- 15 in Wade. Make sure you are familiar with macroscale recrystallization and macroscale separation using a separatory funnel. Introduction: Lidocaine is a member of the Caine family of pharmaceutical local anesthetics. Figure 1 below shows the chemical structure of Lidocaine as well as three other members of this important family of numbing agents. Figure 1. Well-known compounds in the Caine family of local anesthetics NH2 H N CO2CH3 N CO2CH2CH3 N O O Ph N H N O O 2 O Lidocaine Cocaine Novocaine Benzocaine When used correctly, these compounds are very effective at providing local anesthesia, or the numbing feeling you get when you go to the dentist for a filling, for example. These compounds are administered topically, orally, or injected, depending on the situation. Topical creams and ointments that are available over the counter (OTC) generally contain Lidocaine and sometimes Benzocaine. Of the four compounds in Figure 1, Cocaine is not available OTC (hopefully this is obvious) and is not commonly used among the medical community due to its extremely addictive nature. Local anesthetics prevent us from experiencing pain in a small portion of the body, i.e., wherever it is applied. When a local anesthetic is injected directly into the spinal fluid, our entire lower body goes numb. An example of the latter is an epidural given to a woman in labor, most commonly Bupivacaine (structure not shown). A common example of a local anesthetic is the use of Benzocaine in teething gel (Orajel®) for infants. -

RIFM Fragrance Ingredient Safety Assessment, 2-Isopropyl-4- Methylanisole, CAS Registry Number 31574-44-4
Food and Chemical Toxicology 110 (2017) S545eS551 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short review RIFM fragrance ingredient safety assessment, 2-isopropyl-4- methylanisole, CAS Registry Number 31574-44-4 * A.M. Api a, , D. Belsito b, D. Botelho a, D. Browne a, M. Bruze c, G.A. Burton Jr. d, J. Buschmann e, M.L. Dagli f, M. Date a, W. Dekant g, C. Deodhar a, M. Francis a, A.D. Fryer h, K. Joshi a,S.LaCavaa, A. Lapczynski a, D.C. Liebler i,D.O’Brien a, R. Parakhia a,A.Patela, T.M. Penning j, G. Ritacco a, J. Romine a, D. Salvito a, T.W. Schultz k, I.G. Sipes l, Y. Thakkar a, E.H. Theophilus a, A.K. Tiethof a, Y. Tokura m, S. Tsang a, J. Wahler a a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ 07677, USA b Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY 10032, USA c Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE-20502, Sweden d School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI 58109, USA e Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625 Hannover, Germany f University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. Prof. dr. Orlando Marques de Paiva, 87, Sao Paulo CEP 05508-900, Brazil g University of Wuerzburg, Department of Toxicology, Versbacher Str. -

Assessment of Portable HAZMAT Sensors for First Responders
The author(s) shown below used Federal funds provided by the U.S. Department of Justice and prepared the following final report: Document Title: Assessment of Portable HAZMAT Sensors for First Responders Author(s): Chad Huffman, Ph.D., Lars Ericson, Ph.D. Document No.: 246708 Date Received: May 2014 Award Number: 2010-IJ-CX-K024 This report has not been published by the U.S. Department of Justice. To provide better customer service, NCJRS has made this Federally- funded grant report available electronically. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. Department of Justice. Assessment of Portable HAZMAT Sensors for First Responders DOJ Office of Justice Programs National Institute of Justice Sensor, Surveillance, and Biometric Technologies (SSBT) Center of Excellence (CoE) March 1, 2012 Submitted by ManTech Advanced Systems International 1000 Technology Drive, Suite 3310 Fairmont, West Virginia 26554 Telephone: (304) 368-4120 Fax: (304) 366-8096 Dr. Chad Huffman, Senior Scientist Dr. Lars Ericson, Director UNCLASSIFIED This project was supported by Award No. 2010-IJ-CX-K024, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect those of the Department of Justice. This document is a research report submitted to the U.S. Department of Justice. This report has not been published by the Department. Opinions or points of view expressed are those of the author(s) and do not necessarily reflect the official position or policies of the U.S. -

Material Safety Data Sheet
Material Safety Data Sheet Chloroacetyl chloride, 98% ACC# 00956 Section 1 - Chemical Product and Company Identification MSDS Name: Chloroacetyl chloride, 98% Catalog Numbers: AC147290000, AC147290010, AC147290050, AC147291000, AC147292500 Synonyms: Chloracetyl chloride; Chloroacetic acid chloride. Company Identification: Acros Organics N.V. One Reagent Lane Fair Lawn, NJ 07410 For information in North America, call: 800-ACROS-01 For emergencies in the US, call CHEMTREC: 800-424-9300 Section 2 - Composition, Information on Ingredients CAS# Chemical Name Percent EINECS/ELINCS 79-04-9 Chloroacetyl chloride 98 201-171-6 Section 3 - Hazards Identification EMERGENCY OVERVIEW Appearance: colorless to light yellow liquid. Danger! Corrosive. Causes eye and skin burns. Causes digestive and respiratory tract burns. Harmful if swallowed, inhaled, or absorbed through the skin. Lachrymator (substance which increases the flow of tears). Moisture sensitive. Corrosive to metal. Target Organs: Lungs. Potential Health Effects Eye: Lachrymator (substance which increases the flow of tears). Causes eye irritation and burns. Skin: Harmful if absorbed through the skin. Causes severe skin irritation and burns. Ingestion: Harmful if swallowed. Causes gastrointestinal tract burns. Inhalation: Harmful if inhaled. Causes severe irritation of upper respiratory tract with coughing, burns, breathing difficulty, and possible coma. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. Inhalation of high concentrations may cause pulmonary edema. Chronic: Prolonged or repeated exposure may cause lung irritation, chest pain, and pulmonary edema. Section 4 - First Aid Measures Eyes: In case of contact, immediately flush eyes with plenty of water for a t least 15 minutes. Get medical aid immediately. Skin: In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. -

Arenechromium Tricarbonyl Complexes: Conformational
η6 – ARENECHROMIUM TRICARBONYL COMPLEXES: CONFORMATIONAL ANALYSIS, STEREOCONTROL IN NUCLEOPHILIC ADDITION AND APPLICATIONS IN ORGANIC SYNTHESIS by HARINANDINI PARAMAHAMSAN Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy Thesis Advisor: Prof. Anthony J. Pearson Department of Chemistry CASE WESTERN RESERVE UNIVERSITY May 2005 CASE WESTERN RESERVE UNIVERSITY SCHOOL OF GRADUATE STUDIES We hereby approve the dissertation of Harinandini Paramahamsan candidate for the Ph.D. degree*. (signed) Prof. Philip P. Garner (Chair of the Committee, Department of Chemistry, CWRU) Prof. Anthony J. Pearson (Department of Chemistry, CWRU) Prof. Fred L. Urbach (Department of Chemistry, CWRU) Dr. Zwong-Wu Guo (Department of Chemistry, CWRU) Dr. Stuart J. Rowan (Department of Macromolecular Science and Engineering, CWRU) Date: 14th January 2005 *We also certify that written approval has been obtained for any propriety material contained therein. To Amma, Naina & all my Teachers Table of Contents List of Tables………………………………………………………………………..……iv List of Figures…………………………………………………………………….…........vi List of Schemes…………………………………………………………………….….….ix List of Equations………………………………………………………...……….……….xi Acknowledgements………………………………………………………….…..……….xii List of Abbreviations……………………………………………………………………xiv Abstract………………………………………………………………………………….xvi CHAPTER I........................................................................................................................ 1 I.1 Structure and Bonding ........................................................................................... -

Reactions of Aromatic Compounds Just Like an Alkene, Benzene Has Clouds of Electrons Above and Below Its Sigma Bond Framework
Reactions of Aromatic Compounds Just like an alkene, benzene has clouds of electrons above and below its sigma bond framework. Although the electrons are in a stable aromatic system, they are still available for reaction with strong electrophiles. This generates a carbocation which is resonance stabilized (but not aromatic). This cation is called a sigma complex because the electrophile is joined to the benzene ring through a new sigma bond. The sigma complex (also called an arenium ion) is not aromatic since it contains an sp3 carbon (which disrupts the required loop of p orbitals). Ch17 Reactions of Aromatic Compounds (landscape).docx Page1 The loss of aromaticity required to form the sigma complex explains the highly endothermic nature of the first step. (That is why we require strong electrophiles for reaction). The sigma complex wishes to regain its aromaticity, and it may do so by either a reversal of the first step (i.e. regenerate the starting material) or by loss of the proton on the sp3 carbon (leading to a substitution product). When a reaction proceeds this way, it is electrophilic aromatic substitution. There are a wide variety of electrophiles that can be introduced into a benzene ring in this way, and so electrophilic aromatic substitution is a very important method for the synthesis of substituted aromatic compounds. Ch17 Reactions of Aromatic Compounds (landscape).docx Page2 Bromination of Benzene Bromination follows the same general mechanism for the electrophilic aromatic substitution (EAS). Bromine itself is not electrophilic enough to react with benzene. But the addition of a strong Lewis acid (electron pair acceptor), such as FeBr3, catalyses the reaction, and leads to the substitution product. -

RIFM Fragrance Ingredient Safety Assessment, Anisyl Alcohol, CAS Registry T Number 105-13-5 A.M
Food and Chemical Toxicology 134 (2019) 110702 Contents lists available at ScienceDirect Food and Chemical Toxicology journal homepage: www.elsevier.com/locate/foodchemtox Short Review RIFM fragrance ingredient safety assessment, anisyl alcohol, CAS registry T number 105-13-5 A.M. Apia, D. Belsitob, S. Bisertaa, D. Botelhoa, M. Bruzec, G.A. Burton Jr.d, J. Buschmanne, M.A. Cancellieria, M.L. Daglif, M. Datea, W. Dekantg, C. Deodhara, A.D. Fryerh, S. Gadhiaa, L. Jonesa, K. Joshia, A. Lapczynskia, M. Lavellea, D.C. Liebleri, M. Naa, D. O'Briena, A. Patela, T.M. Penningj, G. Ritaccoa, F. Rodriguez-Roperoa, J. Rominea, N. Sadekara, D. Salvitoa, ∗ T.W. Schultzk, F. Siddiqia, I.G. Sipesl, G. Sullivana, , Y. Thakkara, Y. Tokuram, S. Tsanga a Research Institute for Fragrance Materials, Inc., 50 Tice Boulevard, Woodcliff Lake, NJ, 07677, USA b Member Expert Panel, Columbia University Medical Center, Department of Dermatology, 161 Fort Washington Ave., New York, NY, 10032, USA c Member Expert Panel, Malmo University Hospital, Department of Occupational & Environmental Dermatology, Sodra Forstadsgatan 101, Entrance 47, Malmo SE, 20502, Sweden d Member Expert Panel, School of Natural Resources & Environment, University of Michigan, Dana Building G110, 440 Church St., Ann Arbor, MI, 58109, USA e Member Expert Panel, Fraunhofer Institute for Toxicology and Experimental Medicine, Nikolai-Fuchs-Strasse 1, 30625, Hannover, Germany f Member Expert Panel, University of Sao Paulo, School of Veterinary Medicine and Animal Science, Department of Pathology, Av. Prof. dr. Orlando Marques de Paiva, 87, Sao Paulo, CEP 05508-900, Brazil g Member Expert Panel, University of Wuerzburg, Department of Toxicology, Versbacher Str. -

Working with Hazardous Chemicals
A Publication of Reliable Methods for the Preparation of Organic Compounds Working with Hazardous Chemicals The procedures in Organic Syntheses are intended for use only by persons with proper training in experimental organic chemistry. All hazardous materials should be handled using the standard procedures for work with chemicals described in references such as "Prudent Practices in the Laboratory" (The National Academies Press, Washington, D.C., 2011; the full text can be accessed free of charge at http://www.nap.edu/catalog.php?record_id=12654). All chemical waste should be disposed of in accordance with local regulations. For general guidelines for the management of chemical waste, see Chapter 8 of Prudent Practices. In some articles in Organic Syntheses, chemical-specific hazards are highlighted in red “Caution Notes” within a procedure. It is important to recognize that the absence of a caution note does not imply that no significant hazards are associated with the chemicals involved in that procedure. Prior to performing a reaction, a thorough risk assessment should be carried out that includes a review of the potential hazards associated with each chemical and experimental operation on the scale that is planned for the procedure. Guidelines for carrying out a risk assessment and for analyzing the hazards associated with chemicals can be found in Chapter 4 of Prudent Practices. The procedures described in Organic Syntheses are provided as published and are conducted at one's own risk. Organic Syntheses, Inc., its Editors, and its Board of Directors do not warrant or guarantee the safety of individuals using these procedures and hereby disclaim any liability for any injuries or damages claimed to have resulted from or related in any way to the procedures herein. -

1. A. the First Reaction Is a Friedel-Crafts Acylation (FCA), Where the Major Product Is the Para- Isomer (60% Isolated Yield)
1. a. The first reaction is a Friedel-Crafts Acylation (FCA), where the major product is the para- isomer (60% isolated yield). The second reaction is a nitration, where the incoming electrophile (nitronium ion) is directed to the ortho position of the methoxy group. The last reaction is a Wolff-Kishner reduction that converts the acetyl group into an ethyl group. The nitro group does not react under these conditions. OCH OCH OCH OCH3 3 3 3 NO2 NO2 N2H4/KOH CH3COCl/AlCl3 H2SO4/HNO3 0 oC O CH 3 O CH3 CH3 (A) Reaction 1 (B) Reaction 2 (C) Reaction 3 (P) b. The best solvent for the FC-acylation is dichloromethane. Tetrahydrofuran is a fairly strong Lewis base, which would react and deactivate the AlCl3 catalyst. Ethanol would also react with AlCl3 and form alcoholates, which are inactive at FCA catalyst. Dichloromethane is polar enough to dissolve all three compounds but does not form adducts with AlCl3. Thus, aluminum chloride maintains its Lewis acidity. 3+ c. As discussed in lecture, AlCl3*6 H2O is not suitable as catalyst because the Al is not a strong Lewis acid anymore. In addition, larger amounts of water would destroy the acetyl chloride as well (=hydrolysis, CH3COCl + H2O ---- > CH3COOH + HCl). Consequently, the reaction would not proceed in the desired fashion. 3+ OH2 H2O OH2 Al H2O OH2 OH2 d. In order to determine the yield, one has to calculate the number of moles of the reactant and the product. nA = 1.90 mL * 0.996 g/mL/108.14 g/mol = 17.5 mmol nCH3COCl = 2.49 mL * 1.104 g/mL/78.5 g/mol = 35.0 mmol nAlCl3 = 4.67 g/133.5 g/mol = 35.0 mmol Compound (A) is the limiting reagent. -

United States Patent (19) (11) 4,129,595 Suzuki 45) Dec
United States Patent (19) (11) 4,129,595 Suzuki 45) Dec. 12, 1978 (54) PREPARATION OF CHLOROACETYL (56) References Cited CHLORDE PUBLICATIONS E. E. Blaise et al., Comptes Rendus (France), vol. 174, 75 Inventor: Shigeto Suzuki, San Francisco, Calif. pp. 1173-1174, (1922), (Chem. Abstr., vol. 16, 2480). 73) Assignee: Chevron Research Company, San Primary Examiner-Gerald A. Schwartz Francisco, Calif. Attorney, Agent, or Firm-D. A. Newell; John Stoner, Jr. (21) Appl. No.: 891,429 57 ABSTRACT Chloroacetyl chloride is prepared by reacting glycolic (22) Filed: Mar. 29, 1978 acid with thionyl chloride in the presence of nitrogen 51) int. C.’.............................................. CO7C 51/58 containing organic compound orphosphine compound. 52 U.S. C. ................................................ 260/544 Y 58 Field of Search .................................... 260/544 Y 3 Claims, No Drawings 4,129,595 1. 2 glycolic acid with thionyl chloride in the presence of a PREPARATION OF CHLOROACETYL CHLORDE catalytic amount of nitrogen-containing hydrocarbyl organic compound or hydrocarbyl phosphine com BACKGROUND OF THE INVENTION pound at high conversion and yield in accordance with 1. Field of the Invention 5 the present invention. The present invention relates to the preparation of chloroacetyl chloride. More particularly, the invention DETALED DESCRIPTION OF THE relates to the preparation of chloroacetyl chloride by INVENTION reacting glycolic acid with thionyl chloride in the pres The nitrogen-containing hydrocarbyl organic com ence of nitrogen-containing -

Ketene Reactions. I. the Addition of Acid Chlorides
KETENE REACTIONS. I. THE ADDITION OF ACID CHLORIDES TO DIMETHYLKETENE. II. THE CYCLOADDITION OF KETENES TO CARBONYL COMPOUNDS APPROVED: Graduate Committee: Major Professor Committee Member.rr^- Committee Member Committee Member Director of the Department of Chemistry Dean' of the Graduate School Smith, Larry, Ketene Reactions. I. The Addition of Acid Chlorides to DimethyIketene. II. The Cycloaddition of Ketenes to Carbonvl Compounds. Doctor of Philosophy (Chemistry), December, 1970, 63 pp., 3 tables, bibliography, 62 titles. Part I describes the addition of several acid chlorides to dimethylketene. The resulting 3-ketoacid chlorides were isolated and characterized. The reactivities of acid chlorides were found to parallel the parent acid pKa's. A reactivity order of ketenes toward acid chlorides was established. Dimethylketene is more reactive than ketene which is more reactive than diphenylketene. Attempts to effect the addition of an acid halide to a ketene produced by in situ dehydro- halogenation yielded a-halovinyl esters. The addition of acid chlorides to ketenes was concluded to be an ionic process dependent upon the nucleophilic character of the ketene oc- carbon and the polarity of the carbon-chlorine bond in the acid chloride. Part II describes the cycloaddition of several aldo- ketenes to chloral. The ketenes were generated in situ by dehydrohalogenation and dehalogenation of appropriately substituted acyl halides. Both cis- and trans-4-trichloro- Miyl-2-oxetanones are produced in the cycloadditions with the sterically hindered cis isomer predominating. Isomer distributions were determined by vpc or nmr analysis of the reaction solutions. Production of the ketenes by dehalo- genation resulted in enhanced reactivity of the carbonyl compounds.