A Study on the Barbels of a Marine Catfish, Arius Thalassinus (RÜPP.)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

A New Species of Cretaceous Acanthomorph from Canada 15 February 2016, by Sarah Gibson
A new species of Cretaceous acanthomorph from Canada 15 February 2016, by Sarah Gibson to sit flush along the body, helping the fish swim faster by reducing drag, or they can be extended completely out to act as a defense mechanism, in case you are a predator looking for a quick bite. Near the base of the Acanthomorpha phylogenetic tree is a small group of fishes, Polymixiiformes, comprised of a single living genus, Polymixia, more commonly known as the beardfish. This innocuous fish seems harmless, but according to many ichthyologists, Polymixia is just one key to understanding acanthomorph relationships. Unraveling the evolutionary relationships is difficult with a single living genus, but thankfully, polymixiiforms have a fossil record dating back to the Cretaceous, containing an increasing number of taxa as new discoveries are being made, particularly in deposits in North America, where fewer acanthomorph fossils are known compared to Figuring out fish relationships is no small feat. Credit: the more-studied Eastern Tethys Ocean deposits in Near et al. 2013 Europe. For being one of the largest groups of vertebrates, and having one of the richer fossil records among organisms, the relationships of fishes are still hotly debated. Humongous datasets are being compiled that involve molecular (both nuclear and mitochondrial) data, compared and contrasted with thorough morphological analyses. (I'm not going to get into all of it here, simply because of its sheer complexity.) What I am going to get into, however, is the fossil record of one subset of fishes, the acanthomorphs. The stout beardfish, Polymixia nobilis. Credit: Wikipedia Acanthomorphs are teleost fishes that possess true fin spines: a set of prominent, sharp, unsegmented spines in the front portion of their dorsal and/or anal fins, followed by a portion of One such new species was recently described by pliable, segmented, "softer" looking rays. -

A Functional-Morphological Study on the Attachment, Respiration and Feeding Mechanisms in Balitorinae (Balitoridae, Teleostei)
Faculty of Sciences Department of Biology Research group: Evolutionary Morphology of Vertebrates Academic year 2012-2013 A functional-morphological study on the attachment, respiration and feeding mechanisms in Balitorinae (Balitoridae, Teleostei) De Meyer Jens Supervisor: Dr. Tom Geerinckx Thesis submitted to obtain the degree of Tutor: Dr. Tom Geerinckx Master in Biology II © Faculty of Sciences – Evolutionary Morphology of Vertebrates Deze masterproef bevat vertrouwelijk informatie en vertrouwelijke onderzoeksresultaten die toebehoren aan de UGent. De inhoud van de masterproef mag onder geen enkele manier publiek gemaakt worden, noch geheel noch gedeeltelijk zonder de uitdrukkelijke schriftelijke voorafgaandelijke toestemming van de UGent vertegenwoordiger, in casu de promotor. Zo is het nemen van kopieën of het op eender welke wijze dupliceren van het eindwerk verboden, tenzij met schriftelijke toestemming. Het niet respecteren van de confidentiële aard van het eindwerk veroorzaakt onherstelbare schade aan de UGent. Ingeval een geschil zou ontstaan in het kader van deze verklaring, zijn de rechtbanken van het arrondissement Gent uitsluitend bevoegd daarvan kennis te nemen. All rights reserved. This thesis contains confidential information and confidential research results that are property to the UGent. The contents of this master thesis may under no circumstances be made public, nor complete or partial, without the explicit and preceding permission of the UGent representative, i.e. the supervisor. The thesis may under no circumstances be copied or duplicated in any form, unless permission granted in written form. Any violation of the confidential nature of this thesis may impose irreparable damage to the UGent. In case of a dispute that may arise within the context of this declaration, the Judicial Court of© All rights reserved. -

Habitat Preference of an Endangered Hill Stream Catfish Olyra Longicaudata
International Journal of Fisheries and Aquatic Studies 2014; 1(3): 86-93 ISSN: 2347-5129 Habitat Preference of an Endangered Hill Stream IJFAS 2014; 1(3): 86-93 © 2014 IJFAS Catfish Olyra longicaudata (McClelland) From www.fisheriesjournal.com Received: 21-10-2013 Arunachal Pradesh, India Accepted: 25-10-2013 Akash Kachari Akash Kachari, Budhin Gogoi, Rashmi Dutta, Kamhun Aran, Pritha Ghosh, Sudipta Department of Zoology, Rajiv Gandhi Maitra, Samir Bhattacharya, Debangshu N. Das University, Rono Hills, Itanagar- 791112, India ABSTRACT Email: [email protected] Habitat predilection of Olyra longicaudata, was studied systematically in a mountain stream of Dengka village of papumpare district Arunachal Pradesh, India. The enumeration of habitat parameters in-situ Budhin Gogoi revealed that this catfish has the specialized and distinguishable habitat fondness in lotic system. This fish is Department of Zoology, Rajiv Gandhi basically bottom dweller and prefers the places with rocky stream beds, devoid of clay and detrital deposits, University, Rono Hills, Itanagar- 791112, surrounding areas enriched with larval and aquatic insects, crustaceans, annelids, molluscs etc. The fish India prefers shallower and clear running water but cool, soft, slightly alkaline with high level of dissolved oxygen Email: [email protected] and under a good cover of riparian vegetation. The population properties of the species showed a dismal picture with adult count going remarkably down. Rashmi Dutta Department of Zoology, Rajiv Gandhi University, Rono Hills, Itanagar- 791112, Keywords: Olyra longicaudata, Dengka, lotic system, endangered. India Email: [email protected] 1. Introduction Kamhun Aran North- east India is considered as one of the hot spots of freshwater fish biodiversity in the [32] Department of Zoology, Rajiv Gandhi world . -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

The Phylogenetic Relationships of Lampridiform Fishes (Teleostei: Acanthomorpha), Based on a Total-Evidence Analysis of Morphological and Molecular Data E
MOLECULAR PHYLOGENETICS AND EVOLUTION Vol. 10, No. 3, December, pp. 417–425, 1998 ARTICLE NO. FY980532 The Phylogenetic Relationships of Lampridiform Fishes (Teleostei: Acanthomorpha), Based on a Total-Evidence Analysis of Morphological and Molecular Data E. O. Wiley,*,† G. David Johnson,† and Walter Wheaton Dimmick* *Natural History Museum and Department of Systematics and Ecology, University of Kansas, Lawrence, Kansas 66045; and †Division of Fishes, National Museum of Natural History, Washington, DC 20560 Received November 11, 1997; revised March 10, 1998 The Lampridiformes comprise 21 species in 12 gen- We investigated the phylogenetic relationships era and 7 families. The relatively small (40 cm) velifer- among five species of lampridiform fishes, three basal ids and much larger (1.8 m) opahs (Lamprididae) are outgroup species (two aulopiforms and one myctophi- deep bodied. The remaining families are elongate and form), and two species of non-lampridiform acantho- range in body size from the small tube-eyes (Stylephori- morphs (Polymixia and Percopsis) using a combined dae, 31 cm) to the very large Regalecus (17 m). All but parsimony analysis of morphological and molecular the near shore veliferids are oceanic fishes, ranging data. Morphological characters included 28 transfor- mation series obtained from the literature. Molecular from the epipelagic realm to abyssal depths. Lampridi- characters included 223 informative transformation form fishes are characterized by a uniquely protusible series from an aligned 854-base pair fragment of 12S upper jaw apparatus and have been considered a mtDNA and 139 informative transformation series from natural group since Regan (1907) named them. Prior to an aligned 561-base pair fragment of 16S mtDNA. -

Training Manual Series No.15/2018
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by CMFRI Digital Repository DBTR-H D Indian Council of Agricultural Research Ministry of Science and Technology Central Marine Fisheries Research Institute Department of Biotechnology CMFRI Training Manual Series No.15/2018 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual In the frame work of the project: DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals 2015-18 Training Manual This is a limited edition of the CMFRI Training Manual provided to participants of the “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals” organized by the Marine Biotechnology Division of Central Marine Fisheries Research Institute (CMFRI), from 2nd February 2015 - 31st March 2018. Principal Investigator Dr. P. Vijayagopal Compiled & Edited by Dr. P. Vijayagopal Dr. Reynold Peter Assisted by Aditya Prabhakar Swetha Dhamodharan P V ISBN 978-93-82263-24-1 CMFRI Training Manual Series No.15/2018 Published by Dr A Gopalakrishnan Director, Central Marine Fisheries Research Institute (ICAR-CMFRI) Central Marine Fisheries Research Institute PB.No:1603, Ernakulam North P.O, Kochi-682018, India. 2 Foreword Central Marine Fisheries Research Institute (CMFRI), Kochi along with CIFE, Mumbai and CIFA, Bhubaneswar within the Indian Council of Agricultural Research (ICAR) and Department of Biotechnology of Government of India organized a series of training programs entitled “DBT sponsored Three Months National Training in Molecular Biology and Biotechnology for Fisheries Professionals”. -

Euteleostei: Aulopiformes) and the Timing of Deep-Sea Adaptations ⇑ Matthew P
Molecular Phylogenetics and Evolution 57 (2010) 1194–1208 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations ⇑ Matthew P. Davis a, , Christopher Fielitz b a Museum of Natural Science, Louisiana State University, 119 Foster Hall, Baton Rouge, LA 70803, USA b Department of Biology, Emory & Henry College, Emory, VA 24327, USA article info abstract Article history: The divergence times of lizardfishes (Euteleostei: Aulopiformes) are estimated utilizing a Bayesian Received 18 May 2010 approach in combination with knowledge of the fossil record of teleosts and a taxonomic review of fossil Revised 1 September 2010 aulopiform taxa. These results are integrated with a study of character evolution regarding deep-sea evo- Accepted 7 September 2010 lutionary adaptations in the clade, including simultaneous hermaphroditism and tubular eyes. Diver- Available online 18 September 2010 gence time estimations recover that the stem species of the lizardfishes arose during the Early Cretaceous/Late Jurassic in a marine environment with separate sexes, and laterally directed, round eyes. Keywords: Tubular eyes have arisen independently at different times in three deep-sea pelagic predatory aulopiform Phylogenetics lineages. Simultaneous hermaphroditism evolved a single time in the stem species of the suborder Character evolution Deep-sea Alepisauroidei, the clade of deep-sea aulopiforms during the Early Cretaceous. This result indicates the Euteleostei oldest known evolutionary event of simultaneous hermaphroditism in vertebrates, with the Alepisauroidei Hermaphroditism being the largest vertebrate clade with this reproductive strategy. Divergence times Ó 2010 Elsevier Inc. -
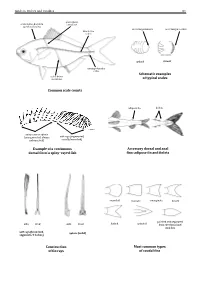
Field Identification Guide to the Living Marine Resources in Kenya
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Phylogeny of Lampridiform Fishes
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 1993 Phylogeny Of Lampridiform Fishes JE Olney Virginia Institute of Marine Science GD Johnson Virginia Institute of Marine Science CC Baldwin Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation Olney, JE; Johnson, GD; and Baldwin, CC, Phylogeny Of Lampridiform Fishes (1993). Bulletin of Marine Science, 52(1), 137-169. https://scholarworks.wm.edu/vimsarticles/1533 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BULLETIN OF MARINE SCIENCE, 52(1): 137-169, 1993 PHYLOGENY OF LAMPRIDIFORM FISHES John E. Olney, G. David Johnson and Carole C. Baldwin ABSTRACT A survey of characters defining the Neoteleostei, Eurypterygii, Ctenosquamata, Acantho- morpha, Paracanthopterygii and Acanthopterygii convincingly places the Lampridiformes within the acanthomorph clade. Lampridiforms are primitive with respect to the Percomorpha but their precise placement among basal acanthomorphs remains unclear. In the absence of a specific sister-group hypothesis, Polymixia. percopsiform and beryciform taxa were used as outgroups in a cladistic analysis of the order. Monophyly of Lampridiformes is supported by four apomorphies; three are correlated modifications related to the evolution ofa unique feeding mechanism in which the maxilla slides forward with the premaxilla during jaw protrusion. The Veliferidae are the sister group of all other lampridiforms. -

The Functioning AFS Network Bill Fisher Reflects As His Year As President of AFS Comes to an End
VOL 37 NO 8 FisheriesAmerican Fisheries Society • www.fisheries.org AUG 2012 Translocating Adult Pacific Lamprey Standardized Monitoring of Fish Populations Demographic Diversity in Natural Resource Science Professions Tilapia in South India Annual Report Inside 03632415(2012)37(8) Specializing in RFID products, expert customer service & biological consulting to the fisheries, wildlife & conservation communities for over 20 years. BIOMARK MULTIPLEXING TRANSCEIVER SYSTEM (IS1001-MTS) Biomark’s Multiplexing Transceiver System FS1001M “MUX” IS1001 “ACN” IS1001-MC (IS1001-MTS) is our newest stationary Input Voltage 24V DC 24V DC range 18–30V DC 24V DC range 18–30V DC reader; providing decoding of ISO Antenna Current 7.0 Ap-p 10.0 Ap-p 10.0 Ap-p 11784/11785 compliant FDX-B & HDX PIT tags. The MTS provides improved Auto-tuning 12 capacitors, 10 capacitors, 10 capacitors, electronically switched electronically switched electronically switched performance, with respect to detection range and number of antennas, over Tags Read 134.2 FDX-B 134.2 FDX-B & HDX 134.2 FDX-B & HDX the current FS1001M stationary reader. Virtual Test Tag Yes, digitally adjustable Yes, digitally adjustable Yes, digitally adjustable The MTS consists of a Master Controller Data Storage 1 x 128 KB: 5350 tags; 2 x 128 KB: 8900 tags; 2 x 128 KB: 15,600 tags; (IS1001-MC) and up to 12 IS1001 reader 146 status reports 151 status reports 151 status reports boards. The IS1001-MC acts as the Antenna Connections 6, multiplexed 1 12, multiplexed/synchronized command and control center for the Communication 1: RS232, DB-9 2 standard USB (Mini-B), 2 standard USB (Mini-B), system, directing each reader when to Ports CAN Bus. -

FIRST RECORD of Polymixia Nobilis LOWE, 1838 (ACTINOPTERYGII: POLYMIXIIDAE) in CEARÁ STATE, BRAZIL
FIRST RECORD OF Polymixia nobilis LOWE, 1838 (ACTINOPTERYGII: POLYMIXIIDAE) IN CEARÁ STATE, BRAZIL Primeiro registro de Polymixia nobilis Lowe, 1838 Arquivos de Ciências do Mar (Actinopterygii: Polymixiidae) no Estado do Ceará, Brasil Carolina Cerqueira de Paiva1, Rodrigo de Salles2, Maria Elisabeth de Araújo3 ABSTRACT The species Polymixia nobilis Lowe, 1838 (Actinopterygii: Polymixiidae) is recorded for the fi rst time in the state of Ceará, Brazil, based on one specimen collected on the Aracati Seamount at a 205 meter depth. Moreover, new meristic and morphologic data are presented. Key words: Polymixia nobilis, fi rst record, taxonomy, geographic distribution, Ceará State. RESUMO A espécie Polyxia nobilis Lowe, 1838 (Actinopterygii: Polimixiidae) é registrada pela promeira vez no Ceará devido à coleta de um exemplar no banco oceânico do Aracati a 205 m de profundidade. São apresentadas também novas informações sobre as variações merísticas e morfológicas da espécie. Palavras-chaves: Polymixia nobilis, primeiro registro, taxonomia, distribuição geográfi ca, Estado do Ceará. 1 Bióloga, Instituto de Cièncias do Mar, Laboratório de Ecologia Animal (LECA). Av. da Abolição 3207, Fortaleza, CE 60165-081. 2 Bolsista da Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico – CAPES/FUNCAP. 3 Professora Associada II, Departamento de Oceanografia – UFPE, Av. Arquitetura, s/n, Campus Universitário, Recife, PE 50740-550. Arq. Ciên. Mar, Fortaleza, 2009, 42(2): 39 - 42 39 INTRODUCTION The aim of the present study was to register the broadening of the geographic distribution of P. Polymixia is the only genus of family nobilis in northeastern Brazil. Polymixiidae and comprises ten species, among which only two are recorded in the Atlantic Ocean: MATERIALS AND METHODS Polymixia nobilis Lowe, 1838 and Polymixia lowei Günther 1859 (Eschmeyer, 2009), commonly known The material was obtained through surveys as beardfi sh (Woods & Sonoda, 1973).