The Phylogenetic Relationships of Lampridiform Fishes (Teleostei: Acanthomorpha), Based on a Total-Evidence Analysis of Morphological and Molecular Data E
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

A New Species of Cretaceous Acanthomorph from Canada 15 February 2016, by Sarah Gibson
A new species of Cretaceous acanthomorph from Canada 15 February 2016, by Sarah Gibson to sit flush along the body, helping the fish swim faster by reducing drag, or they can be extended completely out to act as a defense mechanism, in case you are a predator looking for a quick bite. Near the base of the Acanthomorpha phylogenetic tree is a small group of fishes, Polymixiiformes, comprised of a single living genus, Polymixia, more commonly known as the beardfish. This innocuous fish seems harmless, but according to many ichthyologists, Polymixia is just one key to understanding acanthomorph relationships. Unraveling the evolutionary relationships is difficult with a single living genus, but thankfully, polymixiiforms have a fossil record dating back to the Cretaceous, containing an increasing number of taxa as new discoveries are being made, particularly in deposits in North America, where fewer acanthomorph fossils are known compared to Figuring out fish relationships is no small feat. Credit: the more-studied Eastern Tethys Ocean deposits in Near et al. 2013 Europe. For being one of the largest groups of vertebrates, and having one of the richer fossil records among organisms, the relationships of fishes are still hotly debated. Humongous datasets are being compiled that involve molecular (both nuclear and mitochondrial) data, compared and contrasted with thorough morphological analyses. (I'm not going to get into all of it here, simply because of its sheer complexity.) What I am going to get into, however, is the fossil record of one subset of fishes, the acanthomorphs. The stout beardfish, Polymixia nobilis. Credit: Wikipedia Acanthomorphs are teleost fishes that possess true fin spines: a set of prominent, sharp, unsegmented spines in the front portion of their dorsal and/or anal fins, followed by a portion of One such new species was recently described by pliable, segmented, "softer" looking rays. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

Euteleostei: Aulopiformes) and the Timing of Deep-Sea Adaptations ⇑ Matthew P
Molecular Phylogenetics and Evolution 57 (2010) 1194–1208 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations ⇑ Matthew P. Davis a, , Christopher Fielitz b a Museum of Natural Science, Louisiana State University, 119 Foster Hall, Baton Rouge, LA 70803, USA b Department of Biology, Emory & Henry College, Emory, VA 24327, USA article info abstract Article history: The divergence times of lizardfishes (Euteleostei: Aulopiformes) are estimated utilizing a Bayesian Received 18 May 2010 approach in combination with knowledge of the fossil record of teleosts and a taxonomic review of fossil Revised 1 September 2010 aulopiform taxa. These results are integrated with a study of character evolution regarding deep-sea evo- Accepted 7 September 2010 lutionary adaptations in the clade, including simultaneous hermaphroditism and tubular eyes. Diver- Available online 18 September 2010 gence time estimations recover that the stem species of the lizardfishes arose during the Early Cretaceous/Late Jurassic in a marine environment with separate sexes, and laterally directed, round eyes. Keywords: Tubular eyes have arisen independently at different times in three deep-sea pelagic predatory aulopiform Phylogenetics lineages. Simultaneous hermaphroditism evolved a single time in the stem species of the suborder Character evolution Deep-sea Alepisauroidei, the clade of deep-sea aulopiforms during the Early Cretaceous. This result indicates the Euteleostei oldest known evolutionary event of simultaneous hermaphroditism in vertebrates, with the Alepisauroidei Hermaphroditism being the largest vertebrate clade with this reproductive strategy. Divergence times Ó 2010 Elsevier Inc. -
Field Identification Guide to the Living Marine Resources in Kenya
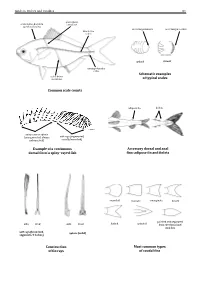
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Phylogeny of Lampridiform Fishes
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 1993 Phylogeny Of Lampridiform Fishes JE Olney Virginia Institute of Marine Science GD Johnson Virginia Institute of Marine Science CC Baldwin Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation Olney, JE; Johnson, GD; and Baldwin, CC, Phylogeny Of Lampridiform Fishes (1993). Bulletin of Marine Science, 52(1), 137-169. https://scholarworks.wm.edu/vimsarticles/1533 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BULLETIN OF MARINE SCIENCE, 52(1): 137-169, 1993 PHYLOGENY OF LAMPRIDIFORM FISHES John E. Olney, G. David Johnson and Carole C. Baldwin ABSTRACT A survey of characters defining the Neoteleostei, Eurypterygii, Ctenosquamata, Acantho- morpha, Paracanthopterygii and Acanthopterygii convincingly places the Lampridiformes within the acanthomorph clade. Lampridiforms are primitive with respect to the Percomorpha but their precise placement among basal acanthomorphs remains unclear. In the absence of a specific sister-group hypothesis, Polymixia. percopsiform and beryciform taxa were used as outgroups in a cladistic analysis of the order. Monophyly of Lampridiformes is supported by four apomorphies; three are correlated modifications related to the evolution ofa unique feeding mechanism in which the maxilla slides forward with the premaxilla during jaw protrusion. The Veliferidae are the sister group of all other lampridiforms. -

FIRST RECORD of Polymixia Nobilis LOWE, 1838 (ACTINOPTERYGII: POLYMIXIIDAE) in CEARÁ STATE, BRAZIL
FIRST RECORD OF Polymixia nobilis LOWE, 1838 (ACTINOPTERYGII: POLYMIXIIDAE) IN CEARÁ STATE, BRAZIL Primeiro registro de Polymixia nobilis Lowe, 1838 Arquivos de Ciências do Mar (Actinopterygii: Polymixiidae) no Estado do Ceará, Brasil Carolina Cerqueira de Paiva1, Rodrigo de Salles2, Maria Elisabeth de Araújo3 ABSTRACT The species Polymixia nobilis Lowe, 1838 (Actinopterygii: Polymixiidae) is recorded for the fi rst time in the state of Ceará, Brazil, based on one specimen collected on the Aracati Seamount at a 205 meter depth. Moreover, new meristic and morphologic data are presented. Key words: Polymixia nobilis, fi rst record, taxonomy, geographic distribution, Ceará State. RESUMO A espécie Polyxia nobilis Lowe, 1838 (Actinopterygii: Polimixiidae) é registrada pela promeira vez no Ceará devido à coleta de um exemplar no banco oceânico do Aracati a 205 m de profundidade. São apresentadas também novas informações sobre as variações merísticas e morfológicas da espécie. Palavras-chaves: Polymixia nobilis, primeiro registro, taxonomia, distribuição geográfi ca, Estado do Ceará. 1 Bióloga, Instituto de Cièncias do Mar, Laboratório de Ecologia Animal (LECA). Av. da Abolição 3207, Fortaleza, CE 60165-081. 2 Bolsista da Fundação Cearense de Apoio ao Desenvolvimento Científico e Tecnológico – CAPES/FUNCAP. 3 Professora Associada II, Departamento de Oceanografia – UFPE, Av. Arquitetura, s/n, Campus Universitário, Recife, PE 50740-550. Arq. Ciên. Mar, Fortaleza, 2009, 42(2): 39 - 42 39 INTRODUCTION The aim of the present study was to register the broadening of the geographic distribution of P. Polymixia is the only genus of family nobilis in northeastern Brazil. Polymixiidae and comprises ten species, among which only two are recorded in the Atlantic Ocean: MATERIALS AND METHODS Polymixia nobilis Lowe, 1838 and Polymixia lowei Günther 1859 (Eschmeyer, 2009), commonly known The material was obtained through surveys as beardfi sh (Woods & Sonoda, 1973). -

Stable Isotopes Reveal Food Web Dynamics of a Data-Poor Deep-Sea Island Slope Community
Food Webs 10 (2017) 22–25 Contents lists available at ScienceDirect Food Webs journal homepage: www.journals.elsevier.com/food-webs Stable isotopes reveal food web dynamics of a data-poor deep-sea island slope community Oliver N. Shipley a,b,c,⁎, Nicholas V.C. Polunin a, Steven P. Newman a,d, Christopher J. Sweeting a, Sam Barker e, Matthew J. Witt e,EdwardJ.Brooksb a School of Marine Science & Technology, Newcastle University, Newcastle Upon Tyne NE1 7RU, UK b Shark Research and Conservation Program, Cape Eleuthera Institute, Eleuthera, PO Box EL-26029, Bahamas, c School of Marine and Atmosphere Sciences, Stony Brook University, 11780, NY, USA d Banyan Tree Marine Lab, Vabbinfaru, Kaafu Atoll, Maldives e The Environment and Sustainability Institute, University of Exeter Penryn Campus, Cornwall TR10 9EZ, UK article info abstract Article history: Deep-sea communities are subject to a growing number of extrinsic pressures, which threaten their structure Received 6 December 2016 and function. Here we use carbon and nitrogen stable isotopes to provide new insights into the community struc- Received in revised form 20 January 2017 ture of a data-poor deep-sea island slope system, the Exuma Sound, the Bahamas. A total of 78 individuals from Accepted 3 February 2017 16 species were captured between 462 m and 923 m depth, and exhibited a broad range of δ13C(9.45‰)andδ15N Available online 12 February 2017 (6.94‰). At the individual-level, δ13C decreased strongly with depth, indicative of shifting production sources, as fi δ15 Keywords: well as potential shifts in community composition, and species-speci c feeding strategies. -

A Study on the Barbels of a Marine Catfish, Arius Thalassinus (RÜPP.)
Japanese Journal of Ichthyology 魚 類 学 雑 誌 Vol.XIV,nos.4/6 第14巻 第4/6号 December 31,1967 1967年12月31日 発 行 A Study on the Barbels of a Marine Catfish, Anus thalassinus (RUPP.) B. G. KAPOOR and S. C. BHARGAVA (Department of Zoology, University of Jodhpur, Jodhpur, India) A great variety of marine and fresh-water fishes possess externally situated appendages, the barbels. These are accessory feeding structures that carry sensory organs and thus seem to play an useful and important role in their daily activities. The number, length and position are extremely variable. The relevant histological findings on barbels are of MENG (1924), BAECKER (1926), SATO (1937a, b), DUCROS (1954), RAFFIN-PEYLOZ (1955), SATO and KAPOOR (1957), NAGAR and MATHUR (1958), SRIVASTAVA and SINHA (1961) and RAJBANSHI (1966). Contributions on gustatory sense in fishes by WUNDER (1927, 1936), EVANS (1940), HASLER (1957) and KONISHI and ZOTTERMAN (1963) are of special interest. In India, the researches on barbels, so far, were confined to fresh-water fishes. From the viewpoint of propagation of knowledge, this study has been conducted on the barbels of a marine catfish, Arius thalassinus (RUPP.) [1 Order•\Cypriniformes; Suborder•\Siluroidei; Family•\Ariidae] [2 Division III; Superorder•\Ostariophysi; Order•\Siluriformes; Family•\Ardiiae]. The male Arius employs the mouth as an oral incubator for developing eggs. The head of Arius, in Bouin's fixative, was kindly supplied by D. SUGANTHARAJ, Tuticorin. Sections (6-8u) were stained with Delafield's haematoxylin in combination with eosin, and also with Mallory's stain. Account of observations Arius thalassinus possesses six barbels-one maxillary and two mandibular pairs (fig. -

Marine Ecology Progress Series 418:1
Vol. 418: 1–15, 2010 MARINE ECOLOGY PROGRESS SERIES Published November 18 doi: 10.3354/meps08814 Mar Ecol Prog Ser OPENPEN ACCESSCCESS FEATURE ARTICLE Characterization of forage fish and invertebrates in the Northwestern Hawaiian Islands using fatty acid signatures: species and ecological groups Jacinthe Piché1,*, Sara J. Iverson1, Frank A. Parrish2, Robert Dollar2 1Department of Biology, Dalhousie University, Halifax, Nova Scotia B3H 4J1, Canada 2Pacific Island Fisheries Science Center, NOAA, 2570 Dole Street, Honolulu, Hawaii 96822, USA ABSTRACT: The fat content and fatty acid (FA) composition of 100 species of fishes and inverte- brates (n = 2190) that are potential key forage spe- cies of the critically endangered monk seal in the Northwestern Hawaiian Islands were determined. For analysis, these species were classified into 47 groups based on a range of shared factors such as taxonomy, diet, ecological subsystem, habitat, and commercial interest. Hierarchical cluster and dis- criminant analyses of the 47 groups using 15 major FAs revealed that groups of species with similar FA composition associated into 5 functional groups: herbivores, planktivores, carnivores (which also in- cluded piscivores and omnivores), crustaceans, and cephalopods. Discriminant analyses performed on the 4 main functional groups separately revealed Reef fish on a bank summit of the Northwestern Hawaiian that herbivores, planktivores, and crustaceans could Islands be readily differentiated on the basis of their FA Image: Raymond Boland signatures, with 97.7, 87.2, and 81.5% of individuals correctly classified, respectively. Classification suc- cess was lower within the carnivores (75.5%), which indicates that some groups of carnivorous species KEY WORDS: Fatty acids · Fishes · Invertebrates · Diet likely exhibit highly similar diets and/or ecology, guild · Trophic relationships · Ecological subsystems · rendering their FA signatures harder to differenti- Hawaiian archipelago · Northwestern Hawaiian Islands ate. -

Proceedings of the United States National Museum
PROCEEDINGS OF THE UNITED STATES NATIONAL MUSEUM issued Iff5(vA, sJ^Ml I'y the SMITHSONIAN INSTITUTION U. S. NATIONAL MUSEUM Vol. 105 Washington: 1955 No. 3356 POPULATIONS OF THE BERYCOID FISH FAMILY POLYMIXIIDAE By Ernest A. Lachner The contributions in this study are the presentation of data mauily from heretofore unreported collections warranting the recognition of thi'ee species of Polymixia, new evidence establishing two species in Atlantic waters, and great extensions of the geogi'aphic distributions of all forms. This family, with a single genus, has had seven nominal forms re- ferred to it. Nineteenth century interest in exploration and natural history accounted for the almost simultaneous descriptions of two species from each of two different geographical areas, Polymixia nobilis Lowe (183G) from Madeira and Nemobrama webbii Valenciennes (1843) from a contiguous area, the Canary Islands, and P. lowei Gunther (1859) and Dinemus venustus Poey (1860) from Cuba. There is dis- agreement in the literature on the exact dates of publication of the descriptions of P. nobilis and A^. webbii. Some authors have given 1838 for the former. Valencienne's "Icththyologie," in Baker-Webb and Berthelot, "Histoire Naturelle des lies Canaries," volume 2, part 2, has been listed under several dates and more generally by some as 1836-44. There is no question of priority, for Valenciennes mentions nobilis in his study. I follow Neave (1940) for my dates of these references. 338380—55 1 189 190 PROCEEDINGS OF THE NATIONAL MUSEUM vol. los The osteology, anatomy, and classification of the berycoid fishes have been generally treated by Starks (1904) and Regan (1911). -

Fishes of the Indian River Lagoon and Adjacent Waters, Florida
FISHES OF THE INDIAN RIVER LAGOON AND ADJACENT WATERS, FLORIDA by R. Grant Gilmore, Jr. Christopher J. Donohoe Douglas W. Cooke Harbor Branch Foundation, Inc. RR 1, Box 196 Fort Pierce, Florida 33450 and David J. Herrema Applied Biology, Inc. 641 DeKalb Industrial Way Decatur, Georgia 30033 Harbor Branch Foundation, Inc. Technical Report No. 41 September 1981 Funding was provided by the Harbor Branch Foundation, Inc. and Florida Power & Light Company, Miami, Florida FISHES OF THE INDIAN RIVER LAGOON AND ADJACENT WATERS, FLORIDA R. Grant Gilmore, Jr. Christopher Donohoe Dougl as Cooke Davi d Herrema INTRODUCTION It is the intent of this presentation to briefly describe regional fish habitats and to list the fishes associated with these habitats in the Indian River lagoon, its freshwater tributaries and the adjacent continental shelf to a depth of 200 m. A brief historical review of other regional ichthyological studies is also given. Data presented here revises the first regional description and checklist of fishes in east central Florida (Gilmore, 1977). The Indian River is a narrow estuarine lagoon system extending from Ponce de Leon Inlet in Vol usia County south to Jupiter Inlet in Palm Beach County (Fig. 1). It lies within the zone of overlap between two well known faunal regimes (i.e. the warm temperate Carolinian and the tropical Caribbean). To the north of the region, Hildebrand and Schroeder (1928), Fowler (1945), Struhsaker (1969), Dahlberg (1971), and others have made major icthyofaunal reviews of the coastal waters of the southeastern United States. McLane (1955) and Tagatz (1967) have made extensive surveys of the fishes of the St.