Stable Isotopes Reveal Food Web Dynamics of a Data-Poor Deep-Sea Island Slope Community
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Early Stages of Fishes in the Western North Atlantic Ocean Volume
ISBN 0-9689167-4-x Early Stages of Fishes in the Western North Atlantic Ocean (Davis Strait, Southern Greenland and Flemish Cap to Cape Hatteras) Volume One Acipenseriformes through Syngnathiformes Michael P. Fahay ii Early Stages of Fishes in the Western North Atlantic Ocean iii Dedication This monograph is dedicated to those highly skilled larval fish illustrators whose talents and efforts have greatly facilitated the study of fish ontogeny. The works of many of those fine illustrators grace these pages. iv Early Stages of Fishes in the Western North Atlantic Ocean v Preface The contents of this monograph are a revision and update of an earlier atlas describing the eggs and larvae of western Atlantic marine fishes occurring between the Scotian Shelf and Cape Hatteras, North Carolina (Fahay, 1983). The three-fold increase in the total num- ber of species covered in the current compilation is the result of both a larger study area and a recent increase in published ontogenetic studies of fishes by many authors and students of the morphology of early stages of marine fishes. It is a tribute to the efforts of those authors that the ontogeny of greater than 70% of species known from the western North Atlantic Ocean is now well described. Michael Fahay 241 Sabino Road West Bath, Maine 04530 U.S.A. vi Acknowledgements I greatly appreciate the help provided by a number of very knowledgeable friends and colleagues dur- ing the preparation of this monograph. Jon Hare undertook a painstakingly critical review of the entire monograph, corrected omissions, inconsistencies, and errors of fact, and made suggestions which markedly improved its organization and presentation. -

A New Species of Cretaceous Acanthomorph from Canada 15 February 2016, by Sarah Gibson
A new species of Cretaceous acanthomorph from Canada 15 February 2016, by Sarah Gibson to sit flush along the body, helping the fish swim faster by reducing drag, or they can be extended completely out to act as a defense mechanism, in case you are a predator looking for a quick bite. Near the base of the Acanthomorpha phylogenetic tree is a small group of fishes, Polymixiiformes, comprised of a single living genus, Polymixia, more commonly known as the beardfish. This innocuous fish seems harmless, but according to many ichthyologists, Polymixia is just one key to understanding acanthomorph relationships. Unraveling the evolutionary relationships is difficult with a single living genus, but thankfully, polymixiiforms have a fossil record dating back to the Cretaceous, containing an increasing number of taxa as new discoveries are being made, particularly in deposits in North America, where fewer acanthomorph fossils are known compared to Figuring out fish relationships is no small feat. Credit: the more-studied Eastern Tethys Ocean deposits in Near et al. 2013 Europe. For being one of the largest groups of vertebrates, and having one of the richer fossil records among organisms, the relationships of fishes are still hotly debated. Humongous datasets are being compiled that involve molecular (both nuclear and mitochondrial) data, compared and contrasted with thorough morphological analyses. (I'm not going to get into all of it here, simply because of its sheer complexity.) What I am going to get into, however, is the fossil record of one subset of fishes, the acanthomorphs. The stout beardfish, Polymixia nobilis. Credit: Wikipedia Acanthomorphs are teleost fishes that possess true fin spines: a set of prominent, sharp, unsegmented spines in the front portion of their dorsal and/or anal fins, followed by a portion of One such new species was recently described by pliable, segmented, "softer" looking rays. -

XIV. Appendices
Appendix 1, Page 1 XIV. Appendices Appendix 1. Vertebrate Species of Alaska1 * Threatened/Endangered Fishes Scientific Name Common Name Eptatretus deani black hagfish Lampetra tridentata Pacific lamprey Lampetra camtschatica Arctic lamprey Lampetra alaskense Alaskan brook lamprey Lampetra ayresii river lamprey Lampetra richardsoni western brook lamprey Hydrolagus colliei spotted ratfish Prionace glauca blue shark Apristurus brunneus brown cat shark Lamna ditropis salmon shark Carcharodon carcharias white shark Cetorhinus maximus basking shark Hexanchus griseus bluntnose sixgill shark Somniosus pacificus Pacific sleeper shark Squalus acanthias spiny dogfish Raja binoculata big skate Raja rhina longnose skate Bathyraja parmifera Alaska skate Bathyraja aleutica Aleutian skate Bathyraja interrupta sandpaper skate Bathyraja lindbergi Commander skate Bathyraja abyssicola deepsea skate Bathyraja maculata whiteblotched skate Bathyraja minispinosa whitebrow skate Bathyraja trachura roughtail skate Bathyraja taranetzi mud skate Bathyraja violacea Okhotsk skate Acipenser medirostris green sturgeon Acipenser transmontanus white sturgeon Polyacanthonotus challengeri longnose tapirfish Synaphobranchus affinis slope cutthroat eel Histiobranchus bathybius deepwater cutthroat eel Avocettina infans blackline snipe eel Nemichthys scolopaceus slender snipe eel Alosa sapidissima American shad Clupea pallasii Pacific herring 1 This appendix lists the vertebrate species of Alaska, but it does not include subspecies, even though some of those are featured in the CWCS. -

Percomorph Phylogeny: a Survey of Acanthomorphs and a New Proposal
BULLETIN OF MARINE SCIENCE, 52(1): 554-626, 1993 PERCOMORPH PHYLOGENY: A SURVEY OF ACANTHOMORPHS AND A NEW PROPOSAL G. David Johnson and Colin Patterson ABSTRACT The interrelationships of acanthomorph fishes are reviewed. We recognize seven mono- phyletic terminal taxa among acanthomorphs: Lampridiformes, Polymixiiformes, Paracan- thopterygii, Stephanoberyciformes, Beryciformes, Zeiformes, and a new taxon named Smeg- mamorpha. The Percomorpha, as currently constituted, are polyphyletic, and the Perciformes are probably paraphyletic. The smegmamorphs comprise five subgroups: Synbranchiformes (Synbranchoidei and Mastacembeloidei), Mugilomorpha (Mugiloidei), Elassomatidae (Elas- soma), Gasterosteiformes, and Atherinomorpha. Monophyly of Lampridiformes is justified elsewhere; we have found no new characters to substantiate the monophyly of Polymixi- iformes (which is not in doubt) or Paracanthopterygii. Stephanoberyciformes uniquely share a modification of the extrascapular, and Beryciformes a modification of the anterior part of the supraorbital and infraorbital sensory canals, here named Jakubowski's organ. Our Zei- formes excludes the Caproidae, and characters are proposed to justify the monophyly of the group in that restricted sense. The Smegmamorpha are thought to be monophyletic principally because of the configuration of the first vertebra and its intermuscular bone. Within the Smegmamorpha, the Atherinomorpha and Mugilomorpha are shown to be monophyletic elsewhere. Our Gasterosteiformes includes the syngnathoids and the Pegasiformes -

The Phylogenetic Relationships of Lampridiform Fishes (Teleostei: Acanthomorpha), Based on a Total-Evidence Analysis of Morphological and Molecular Data E
MOLECULAR PHYLOGENETICS AND EVOLUTION Vol. 10, No. 3, December, pp. 417–425, 1998 ARTICLE NO. FY980532 The Phylogenetic Relationships of Lampridiform Fishes (Teleostei: Acanthomorpha), Based on a Total-Evidence Analysis of Morphological and Molecular Data E. O. Wiley,*,† G. David Johnson,† and Walter Wheaton Dimmick* *Natural History Museum and Department of Systematics and Ecology, University of Kansas, Lawrence, Kansas 66045; and †Division of Fishes, National Museum of Natural History, Washington, DC 20560 Received November 11, 1997; revised March 10, 1998 The Lampridiformes comprise 21 species in 12 gen- We investigated the phylogenetic relationships era and 7 families. The relatively small (40 cm) velifer- among five species of lampridiform fishes, three basal ids and much larger (1.8 m) opahs (Lamprididae) are outgroup species (two aulopiforms and one myctophi- deep bodied. The remaining families are elongate and form), and two species of non-lampridiform acantho- range in body size from the small tube-eyes (Stylephori- morphs (Polymixia and Percopsis) using a combined dae, 31 cm) to the very large Regalecus (17 m). All but parsimony analysis of morphological and molecular the near shore veliferids are oceanic fishes, ranging data. Morphological characters included 28 transfor- mation series obtained from the literature. Molecular from the epipelagic realm to abyssal depths. Lampridi- characters included 223 informative transformation form fishes are characterized by a uniquely protusible series from an aligned 854-base pair fragment of 12S upper jaw apparatus and have been considered a mtDNA and 139 informative transformation series from natural group since Regan (1907) named them. Prior to an aligned 561-base pair fragment of 16S mtDNA. -

Euteleostei: Aulopiformes) and the Timing of Deep-Sea Adaptations ⇑ Matthew P
Molecular Phylogenetics and Evolution 57 (2010) 1194–1208 Contents lists available at ScienceDirect Molecular Phylogenetics and Evolution journal homepage: www.elsevier.com/locate/ympev Estimating divergence times of lizardfishes and their allies (Euteleostei: Aulopiformes) and the timing of deep-sea adaptations ⇑ Matthew P. Davis a, , Christopher Fielitz b a Museum of Natural Science, Louisiana State University, 119 Foster Hall, Baton Rouge, LA 70803, USA b Department of Biology, Emory & Henry College, Emory, VA 24327, USA article info abstract Article history: The divergence times of lizardfishes (Euteleostei: Aulopiformes) are estimated utilizing a Bayesian Received 18 May 2010 approach in combination with knowledge of the fossil record of teleosts and a taxonomic review of fossil Revised 1 September 2010 aulopiform taxa. These results are integrated with a study of character evolution regarding deep-sea evo- Accepted 7 September 2010 lutionary adaptations in the clade, including simultaneous hermaphroditism and tubular eyes. Diver- Available online 18 September 2010 gence time estimations recover that the stem species of the lizardfishes arose during the Early Cretaceous/Late Jurassic in a marine environment with separate sexes, and laterally directed, round eyes. Keywords: Tubular eyes have arisen independently at different times in three deep-sea pelagic predatory aulopiform Phylogenetics lineages. Simultaneous hermaphroditism evolved a single time in the stem species of the suborder Character evolution Deep-sea Alepisauroidei, the clade of deep-sea aulopiforms during the Early Cretaceous. This result indicates the Euteleostei oldest known evolutionary event of simultaneous hermaphroditism in vertebrates, with the Alepisauroidei Hermaphroditism being the largest vertebrate clade with this reproductive strategy. Divergence times Ó 2010 Elsevier Inc. -
Field Identification Guide to the Living Marine Resources in Kenya
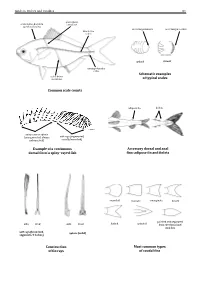
Guide to Orders and Families 81 lateral line scales above scales before dorsal fin outer margin smooth outer margin toothed (predorsal scales) lateral–line 114 scales cycloid ctenoidِّ scales circumpeduncular Schematic examples lateral line of typical scales scales below Common scale counts adipose fin finlets soft rays (segmented, spinyunbranched) rays or spines usually branched) (unsegmented, always Example of a continuous Accessory dorsal and anal dorsal fin of a spiny–rayed fish fins: adipose fin and finlets rounded truncate emarginate lunate side front side front from the dorsal and pointed and separated forked pointed soft rays (branched, spines (solid) segments, 2 halves) anal fins Construction Most common types of fin rays of caudal fins 82 Bony Fishes GUIDE TO ORDERS AND FAMILIES Order ELOPIFORMES – Tarpons and allies Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 23–25 branchiostegal rays; upper jaw extending past eye; tip of snout not overhanging mouth; colour silvery. ELOPIDAE Page 121 very small scales Ladyfishes To 90 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head large mouth gular plate MEGALOPIDAE Page 121 last ray long Tarpons large scales To 55 cm. Coastal marine waters and estuaries; pelagic. A single species included in the Guide to Species.underside of head gular plate Order ALBULIFORMES – Bonefishes Fin spines absent; a single dorsal fin located above middle of body; pelvic fins in abdominal position; lateral line present; 6–16 branchiostegal rays; upper jaw not extending as far as front of eye; tip of snout overhanging mouth; colour silvery. -

Annotated Checklist of the Fish Species (Pisces) of La Réunion, Including a Red List of Threatened and Declining Species
Stuttgarter Beiträge zur Naturkunde A, Neue Serie 2: 1–168; Stuttgart, 30.IV.2009. 1 Annotated checklist of the fish species (Pisces) of La Réunion, including a Red List of threatened and declining species RONALD FR ICKE , THIE rr Y MULOCHAU , PA tr ICK DU R VILLE , PASCALE CHABANE T , Emm ANUEL TESSIE R & YVES LE T OU R NEU R Abstract An annotated checklist of the fish species of La Réunion (southwestern Indian Ocean) comprises a total of 984 species in 164 families (including 16 species which are not native). 65 species (plus 16 introduced) occur in fresh- water, with the Gobiidae as the largest freshwater fish family. 165 species (plus 16 introduced) live in transitional waters. In marine habitats, 965 species (plus two introduced) are found, with the Labridae, Serranidae and Gobiidae being the largest families; 56.7 % of these species live in shallow coral reefs, 33.7 % inside the fringing reef, 28.0 % in shallow rocky reefs, 16.8 % on sand bottoms, 14.0 % in deep reefs, 11.9 % on the reef flat, and 11.1 % in estuaries. 63 species are first records for Réunion. Zoogeographically, 65 % of the fish fauna have a widespread Indo-Pacific distribution, while only 2.6 % are Mascarene endemics, and 0.7 % Réunion endemics. The classification of the following species is changed in the present paper: Anguilla labiata (Peters, 1852) [pre- viously A. bengalensis labiata]; Microphis millepunctatus (Kaup, 1856) [previously M. brachyurus millepunctatus]; Epinephelus oceanicus (Lacepède, 1802) [previously E. fasciatus (non Forsskål in Niebuhr, 1775)]; Ostorhinchus fasciatus (White, 1790) [previously Apogon fasciatus]; Mulloidichthys auriflamma (Forsskål in Niebuhr, 1775) [previously Mulloidichthys vanicolensis (non Valenciennes in Cuvier & Valenciennes, 1831)]; Stegastes luteobrun- neus (Smith, 1960) [previously S. -

10 Taxonomy, Biology and Distribution of Deep Sea Shrimps
Taxonomy, Biology and 10 Distribution of Deep Sea Shrimps Rekha Devi Chakraborty Crustacean Fisheries Division Shellfish systematics is the most unique one in fisheries science in view of its importance and implications in diversity. The systematic zoology is the science that discovers names, determines relationships, classifies and studies the evolution of living organisms. It is an important branch in biology and is considered to be one of the major subdivisions of biology having a broader base than genetics, biochemistry and physiology. The shellfish includes two highly diversified phyla i.e. phylum Arthropoda and phylum Mollusca. These two groups are named as shellfishes because of the presence of exoskeleton made of chitin in arthropods and shells made of calcium in molluscs. These two major phyla are invertebrates. They show enormous diversity in their morphology, in the habitats they occupy and in their biology. Phylum Arthropoda includes economically important groups such as lobsters, shrimps, crabs. Taxonomical study reveals numerous interesting phenomena in shellfish phylogeny and the study is most indispensable for the correct identification of candidate species for conservation and management of our fishery resources and aquaculture practices. On the whole taxonomic study on shellfishes furnishes the urgently needed information about species and it cultivates a way of thinking and approaching of all biological problems, which are much needed for the balance and well being of shellfish biology as a whole. Training Manual on Species Identification Shrimp resources are available both from inshore and from offshore waters. As the fish resource from inshore waters remained static during the last two decades, fishing pattern underwent several changes in the previous decade, leading to the exploitation of deep sea resources either with deployment of large sized vessels or modified medium/small sized vessels. -

Behaviour and Habitat Utilisation of Seven Demersal Fish Species on the Bay of Biscay Continental Slope, NE Atlantic
MARINE ECOLOGY PROGRESS SERIES Vol. 257: 223–232, 2003 Published August 7 Mar Ecol Prog Ser Behaviour and habitat utilisation of seven demersal fish species on the Bay of Biscay continental slope, NE Atlantic Franz Uiblein1,*, Pascal Lorance2, Daniel Latrouite2 1Institute of Zoology, University of Salzburg, Hellbrunnerstr. 34, 5020 Salzburg, Austria 2IFREMER, Technopole Brest-Iroise, BP 70, 29280 Plouzané, France ABSTRACT: Much is known in very broad terms about the distribution of deep-sea fishes, but infor- mation on fine-scale habitat selection and behaviour in the largest living space on earth is still rare. Based on video sequences from 4 dives performed with the manned submersible ‘Nautile’ at depths between 400 and 2000 m in the Bay of Biscay, NE Atlantic, we studied the behaviour of co-occurring slope-dwelling deep-sea fishes. Five different habitats were identified according to depth range or topographical and hydrological characteristics. For each fish species or genus that could be identi- fied, estimates of absolute abundance were provided. The most frequently occurring species, round- nose grenadier Coryphaenoides rupestris, blackmouth catshark Galeus melastomus, bluemouth Helicolenus dactylopterus dactylopterus, orange roughy Hoplostethus atlanticus, North Atlantic codling Lepidion eques, greater forkbeard Phycis blennoides, and northern cutthroat eel Synapho- branchus kaupi were quantitatively compared with respect to the relative frequencies of disturbance responses to the submersible, locomotion behaviour, and vertical positioning above the bottom. Clear variations in behaviour and abundance among species and habitats were found, reflecting both species-specific and flexible adjustment to small-scale spatial and temporal variability on the Bay of Biscay continental slope. These results have important implications for the development of sustainable deep-water fisheries management. -

Phylogeny of Lampridiform Fishes
W&M ScholarWorks VIMS Articles Virginia Institute of Marine Science 1993 Phylogeny Of Lampridiform Fishes JE Olney Virginia Institute of Marine Science GD Johnson Virginia Institute of Marine Science CC Baldwin Virginia Institute of Marine Science Follow this and additional works at: https://scholarworks.wm.edu/vimsarticles Part of the Aquaculture and Fisheries Commons, and the Marine Biology Commons Recommended Citation Olney, JE; Johnson, GD; and Baldwin, CC, Phylogeny Of Lampridiform Fishes (1993). Bulletin of Marine Science, 52(1), 137-169. https://scholarworks.wm.edu/vimsarticles/1533 This Article is brought to you for free and open access by the Virginia Institute of Marine Science at W&M ScholarWorks. It has been accepted for inclusion in VIMS Articles by an authorized administrator of W&M ScholarWorks. For more information, please contact [email protected]. BULLETIN OF MARINE SCIENCE, 52(1): 137-169, 1993 PHYLOGENY OF LAMPRIDIFORM FISHES John E. Olney, G. David Johnson and Carole C. Baldwin ABSTRACT A survey of characters defining the Neoteleostei, Eurypterygii, Ctenosquamata, Acantho- morpha, Paracanthopterygii and Acanthopterygii convincingly places the Lampridiformes within the acanthomorph clade. Lampridiforms are primitive with respect to the Percomorpha but their precise placement among basal acanthomorphs remains unclear. In the absence of a specific sister-group hypothesis, Polymixia. percopsiform and beryciform taxa were used as outgroups in a cladistic analysis of the order. Monophyly of Lampridiformes is supported by four apomorphies; three are correlated modifications related to the evolution ofa unique feeding mechanism in which the maxilla slides forward with the premaxilla during jaw protrusion. The Veliferidae are the sister group of all other lampridiforms. -

Remote Camera and Trapping Survey of the Deep-Water Shrimps Heterocarpus Laevigatus and H
Remote Camera and Trapping Survey of the Deep-water Shrimps Heterocarpus laevigatus and H. ensifer and the Geryonid Crab Chaceon granulatus in Palau W. B. SAUNDERS and LEE C. HASTIE Introduction persal, and depth ranges, as well as scale deep-water shrimp fishery in 3 population characteristics such as size Palau (Saunders et aI., 1989 ). Deep-water bottom-dwelling com distribution, sex ratios, etc. More so Procedures munities of the Indo-Pacific are not phisticated analyses of larger trapping well known, owing to their relative data bases have been used to describe Trapping inaccessibility. Most available infor reproductive biology, growth, and mation has been derived from trap mortality (Dailey and Ralston, 1986), A total of 103 traps was set between ping-based surveys which, for the and to calculate potential exploitable 170-900 m depth at five sites around most part, have been pilot efforts di biomass and sustainable yields under Palau (Fig. I) during May-October rected at evaluating economic poten intensive fishing pressure (Polovina et 1987 and 1988. The following trap de tial of deep-water shrimps (King, aI., 1985; Ralston, 1986; Moffitt and signs were used during the survey: I) 1980, 1982, 1984; Struhsaker and Polovina, 1987; Tagami and Ralston, A small collapsible trap (60 x 40 x 20 Aasted, 1974; Gooding, 1984). These 1988). Only recently has it been lo cm); 2) a large pyramidal trap (2 m surveys have generated information on gistically possible to attempt to study square at the base x 1.5 m high); 3) a species identifications, geographic dis- the deepwater habitat directly, and sev traditional fish trap design (1.5 x 1 x 1 eral recent efforts using a submers , m); 4) a covered box trap (1.5 x 0.5 x ible show much promise (Ralston et 0.5 m); 5) a small box-shaped trap (1 x W.