Alternaria Brassicicola – Brassicaceae Pathosystem: Insights Into the Infection Process and Resistance Mechanisms Under Optimized Artificial Bio-Assay
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
THE EVOLUTION of SEED MORPHOLOGY in DOMESTICATED Chenopodium: an ARCHAEOLOGICAL CASE STUDY
]. Ethnobiol. 13(2):149-169 Winter 1993 THE EVOLUTION OF SEED MORPHOLOGY IN DOMESTICATED Chenopodium: AN ARCHAEOLOGICAL CASE STUDY KRISTEN }. GREMILLION Department of Anthropology The Ohio Stute University Columbus, OH 43210-1364 ABSTRACf.-A large body of data on several key morphological characters has been compiled through examination of collections of archaeological Chenopodium from eastern North America. Contrary to expectations based on change in certain other seed crops, the patterns of variation observed in Chenopodium do not reflect a gradual evolution of seed morphology away from the wild type. Evidence for decreasing levels of morphological variability in the evolving domesticate is like wise minimal. These findings demonstrate that the rate and character of crop evolution as revealed in the archaeological record can be expected to vary consid erably among taxa. RESUMEN.-Se ha compilado un extenso ouerpo de datos sobre varios carac teres morfol6gicos clave mediante el examen de colecciones de Chenopodium arqueol6gico del este de Norteamerica. Contrariamente a las expectativas basadas en el cambio en ciertos otms cultivos de semilla, los patrones de variaci6n obser vados en Chenopodium no reflejan una evoluci6n gradual de la morfologia de las semillas en credente distancia del tipo silvestre. La evidencia de niveles decre cientes de variabilidad morfol6gica en la especie domesticada en evoluci6n es asimismo minima. Estos resultados demuestran que puede esperarse que la 13sa y el caracter de la evoluci6n de los cultivos, tal y como se revela en el registro arqueol6gico, varien considerablemente entre taxa distintos. REsUME.-Un large ensemble de donnees concernant plusieurs characteres mor phologiques importants a ete recueilli en examinant des collections de Cheno podium de I'est de I' Amerique. -

Alternaria Brassicicola)
Int.J.Curr.Microbiol.App.Sci (2020) 9(8): 2553-2559 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 9 Number 8 (2020) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2020.908.292 In-vivo Management of Alternaria Leaf Spot of Cabbage (Alternaria brassicicola) Dwarkadas T. Bhere*, K. M. Solanke, Amrita Subhadarshini, Shashi Tiwari and Mohan K. Narode Department of Plant Pathology, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, (U. P), India *Corresponding author ABSTRACT K e yw or ds An experiment was conducted for in-vivo management of Alternaria leaf spot of Cabbage. Alternaria leaf spot, The experiment was analyzed by using RBD (randomized block design) with three 2 Cabbage, replications in a plot size 2x2m . Eight treatments were taken i.e. Neem oil, Eucalyptus oil, Eucalyptus oil and Clove oil, Trichoderma viride, Neem oil + Trichoderma viride, Eucalyptus oil + Clove oil, Trichoderma viride, Clove oil + Trichoderma viride along with the control. Observations Trichoderma viride, were recorded at disease intensity 30, 45 and 60 (days after Transplanting), plant growth Neem oil parameters such a yield (q/ha). Experiment revealed that Neem oil significantly reduced the Alternaria leaf spot of Cabbage, where among the use Neem oil seedling treatment @ Article Info 5% increased the yield. The maximum cost benefit ratio was recorded by Neem oil (1:3.26) Thus according to experimental finding and results discussed in the earlier Accepted: chapter, it is concluded that Neem oil reduced the Alternaria leaf spot of Cabbage, where 22 July 2020 among the Neem oil seedling application found maximum yield was significantly superior Available Online: 10 August 2020 as compare to other treatments. -

Infection Cycle of Alternaria Brassicicola on Brassica Oleracea Leaves Under Growth Room Conditions
Plant Pathology (2018) 67, 1088–1096 Doi: 10.1111/ppa.12828 Infection cycle of Alternaria brassicicola on Brassica oleracea leaves under growth room conditions V. K. Macioszeka, C. B. Lawrenceb and A. K. Kononowicza* aDepartment of Genetics, Plant Molecular Biology and Biotechnology, Faculty of Biology and Environmental Protection, University of Lodz, 90-237 Lodz, Poland; and bDepartment of Biological Sciences, Virginia Tech, Blacksburg, VA 24061, USA Development of the necrotrophic fungus Alternaria brassicicola was evaluated during infection of three cabbage vari- eties: Brassica oleracea var. capitata f. alba ‘Stone Head’ (white cabbage), B. oleracea var. capitata f. rubra ‘Langedi- jker Dauer’ (red cabbage) and B. oleracea var. capitata f. sabauda ‘Langedijker Dauerwirsing’ (Savoy cabbage). Following inoculation of cabbage leaves, conidial germination, germ tube growth, and appressorium formation were analysed during the first 24 h of infection. Differences in the dynamics of fungal development on leaves were observed, e.g. approximately 40% of conidia germinated on Savoy cabbage leaves at 4 h post-inoculation (hpi) while only 20% germinated on red and white cabbage leaves. Leaf penetration on the three cabbage varieties mainly occurred through appressoria, rarely through stomata. Formation of infection cushions was found exclusively on red cabbage. Appresso- ria were first observed on red cabbage leaves at 6 hpi, and on white and Savoy cabbage leaves at 8 hpi. Conidiogenesis occurred directly from mature conidia at an early stage of fungal development (10 hpi), but later (48 hpi) it occurred through conidiophores. Disease progress and changes in the morphology of leaf surfaces were also observed. At the final 120 hpi measurement point, necroses on all investigated varieties were approximately the same size. -

Nature and Effect of Alternaria Spp. Complex from Wheat Grain on Germination and Disease Transmission
Pak. J. Bot., 45(5): 1817-1824, 2013. NATURE AND EFFECT OF ALTERNARIA SPP. COMPLEX FROM WHEAT GRAIN ON GERMINATION AND DISEASE TRANSMISSION ANALÍA E. PERELLÓ1,2* AND SILVINA LARRÁN1 1CIDEFI (Centro de Investigaciones de Fitopatología) y Cátedra de Fitopatología 2CONICET-Facultad de Ciencias Agrarias y Forestales de la Universidad Nacional de La Plata, Calle 60 y 119 (1900) La Plata, Buenos Aires, Argentina. *Corresponding author’s e-mail: anaperello2@ yahoo.com.ar Abstract Diseases caused by Alternaria sp. are among the most common diseases of crops throughout the world. Alternaria sp. is a common component of the flora of wheat seed. Although isolation of Alternaria sp. from wheat (Triticum aestivum) seed has been reported in Argentina, development of the Alternaria blight in plants from infected seeds has not been demonstrated experimentally. Seed transmission of strains belonging to Alternaria tenuissima, A. alternata, A. infectoria, A. triticina, A. chlamydospora and related genera like Embellisia and Ulocladium sp. on wheat were investigated in the Argentinean growing area, on wheat cultivars Klein Escorpión and Buck Poncho. A. tenuissima was the dominant fungus in black pointed kernels. Transmission of all 42 seed-borne members of Alternaria complex from seeds to seedlings artificially inoculated was detected by trays seedling symptoms test. Among the fungi tested most isolates of Alternaria, Embellisia sp. and Ulocladium sp. produced distinct seed rot and seedling infection symptoms. This confirmed the seed-borne nature of these fungi. In each wheat cultivar tested inoculated seeds appreciably reduced their germination. The emerging coleoptile is externally infected by hyphal growth from the infected pericarp. -

Climate Change and Cultural Response in the Prehistoric American Southwest
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln USGS Staff -- Published Research US Geological Survey Fall 2009 Climate Change and Cultural Response In The Prehistoric American Southwest Larry Benson U.S. Geological Survey, [email protected] Michael S. Berry Bureau of Reclamation Follow this and additional works at: https://digitalcommons.unl.edu/usgsstaffpub Benson, Larry and Berry, Michael S., "Climate Change and Cultural Response In The Prehistoric American Southwest" (2009). USGS Staff -- Published Research. 725. https://digitalcommons.unl.edu/usgsstaffpub/725 This Article is brought to you for free and open access by the US Geological Survey at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in USGS Staff -- Published Research by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. CLIMATE CHANGE AND CULTURAL RESPONSE IN THE PREHISTORIC AMERICAN SOUTHWEST Larry V. Benson and Michael S. Berry ABSTRACT Comparison of regional tree-ring cutting-date distributions from the southern Col- orado Plateau and the Rio Grande region with tree-ring-based reconstructions of the Palmer Drought Severity Index (PDSI) and with the timing of archaeological stage transitions indicates that Southwestern Native American cultures were peri- odically impacted by major climatic oscillations between A.D. 860 and 1600. Site- specifi c information indicates that aggregation, abandonment, and out-migration from many archaeological regions occurred during several widespread mega- droughts, including the well-documented middle-twelfth- and late-thirteenth- century droughts. We suggest that the demographic response of southwestern Native Americans to climate variability primarily refl ects their dependence on an inordinately maize-based subsistence regimen within a region in which agricul- ture was highly sensitive to climate change. -
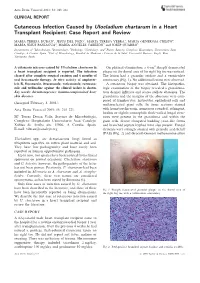
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Identification and Characterization of Alternaria Species Causing Leaf Spot
Eur J Plant Pathol (2017) 149:401–413 DOI 10.1007/s10658-017-1190-0 Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production Ilenia Siciliano & Giovanna Gilardi & Giuseppe Ortu & Ulrich Gisi & Maria Lodovica Gullino & Angelo Garibaldi Accepted: 22 February 2017 /Published online: 11 March 2017 # Koninklijke Nederlandse Planteziektenkundige Vereniging 2017 Abstract Alternaria species are common pathogens of Keywords Crucifers . Toxins . Leaf spot . Tenuazonic fruit and vegetables able to produce secondary metabo- acid lites potentially affecting human health. Twenty-nine isolates obtained from cabbage, cauliflower, wild and cultivated rocket were characterized and identified Introduction based on sporulation pattern and virulence; the phylo- β genetic analysis was based on the -tubulin gene. Most Alternaria species are saprophytes and ubiquitous Isolates were identified as A. alternata, A. tenuissima, in the environment, however some are plant pathogenic, A. arborescens, A. brassicicola and A. japonica. inducing diseases on a large variety of economically Pathogenicity was evaluated on plants under greenhouse important crops like cereals, oil-crops, vegetables and conditions. Two isolates showed low level of virulence fruits (Pitt and Hocking, 1997). Most Alternaria spp. on cultivated rocket while the other isolates showed produce chains of conidia with transverse and longitu- medium or high level of virulence. Isolates were also dinal septa with a tapering apical cell. Conidial size, characterized for their mycotoxin production on a mod- presence and size of a beak, the pattern of catenation ified Czapek-Dox medium. Production of the five and longitudinal and transverse septation are key taxo- Alternaria toxins, tenuazonic acid, alternariol, nomic features for this genus (Joly 1964; Ellis 1971 and alternariol monomethyl ether, altenuene and tentoxin 1976, Simmons 1992). -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -

Sieving Methodology for Recovery of Large Cultigen Pollen
An improved methodology for the recovery of Zea mays and other large crop pollen, with implications for environmental archaeology in the Neotropics Article Accepted Version Whitney, B. S., Rushton, E. A. C., Carson, J. F., Iriarte, J. and Mayle, F. E. (2012) An improved methodology for the recovery of Zea mays and other large crop pollen, with implications for environmental archaeology in the Neotropics. The Holocene, 22 (10). pp. 1087-1096. ISSN 0959-6836 doi: https://doi.org/10.1177/0959683612441842 Available at http://centaur.reading.ac.uk/32925/ It is advisable to refer to the publisher’s version if you intend to cite from the work. See Guidance on citing . To link to this article DOI: http://dx.doi.org/10.1177/0959683612441842 Publisher: Sage Publications All outputs in CentAUR are protected by Intellectual Property Rights law, including copyright law. Copyright and IPR is retained by the creators or other copyright holders. Terms and conditions for use of this material are defined in the End User Agreement . www.reading.ac.uk/centaur CentAUR Central Archive at the University of Reading Reading’s research outputs online An improved methodology for the recovery of Zea mays and other large crop pollen, with implications for environmental archaeology in the Neotropics Bronwen S. Whitney1*, Elizabeth A. C. Rushton2, John F. Carson3,1, Jose Iriarte4, and Francis E. Mayle3 1School of Geosciences, The University of Edinburgh, Drummond St., Edinburgh EH8 9XP, UK 2School of Geography, The University of Nottingham, University Park, Nottingham NG7 2RD, UK 3School of Archaeology, Geography and Environmental Science, The University of Reading, Whiteknights, Reading RG6 6AB, UK 4Department of Archaeology, College of Humanities, University of Exeter, Exeter EX4 4QE, UK *Corresponding author, email: [email protected] tel: +44(0)131 650 9140 fax: +44(0)131 650 2524 A. -

Managing Alternaria Brown Spot by L.W
Managing Alternaria brown spot By L.W. Timmer, R. F. Reis, S.N. Mondal and N.A. Peres anker and greening may be problem for production of Fortune tan - the diseases that are on gerine in Spain. More recently, we Fig. 1. Alternaria brown spot lesions everyone’s minds, but we have confirmed the presence of the on a Minneola tangelo leaf. Note the still have to deal with the disease in Peru, where it is severe on characteristic necrosis running up Cmany everyday problems of produc - Minneolas, and in Iran, where it the veins. tion. Alternaria brown spot is the most causes problems mostly for Fortune for a few days on the grove floor, but serious disease of many popular tan - and Page production. then ceases as the leaves decay. Spores gerines and tangerine hybrids such as Tangerine cultivars, including Min - can also be produced on fruit and Minneola and Orlando tangelos, Mur - neolas, Orlandos, Novas, Lees, twigs, but are relatively few compared cotts, Sunburst, Novas and others. Ponkan, Murcotts, Dancy and many to the production on leaves. However, This disease probably requires more others, that are susceptible to ABS lesions on fruit and twigs may be im - intense management than any of the carry a gene for susceptibility to the portant in the overw-inter survival of other fungal diseases. toxin. That gene is dominant and all of the pathogen. Alternaria brown spot (ABS) is the offspring of susceptible parents are In the 2004-05 season, Alternaria caused by the fungus Alternaria alter - also susceptible. Many cultivars devel - pressure was very low and growers nata . -

Archaeological Evidence of Aboriginal Cultigen Use in Late Nineteenth and Early Twentieth Century Death Valley, California
Journal of Ethnobiology 17(2):267-282 Winter 1997 ARCHAEOLOGICAL EVIDENCE OF ABORIGINAL CULTIGEN USE IN LATE NINETEENTH AND EARLY TWENTIETH CENTURY DEATH VALLEY, CALIFORNIA ROBERT M. YOHE, II Archaeological Survey of Idaho Idaho State Historical Society Boise, Idaho 83702 ABSTRACT.-During archaeological test excavations in two rockshelters in Death Valley, California, two storage features were unearthed which were found to contain numerous perishable artifacts and foodstuffs. In addition to seed remains of indigenous species, including mesquite and pinon, several seeds of introduced cultigens were recovered from within the features, including melon, squash, and beans. The feature containing the greatest number of domesticate seeds appears to date to the late nineteenth and/or early twentieth century and represents the first reported archaeological evidence of Shoshoni horticulture in the southwestem Great Basin. RESUMEN.-Durante excavaciones arqueologicas preliminares en dos refugios de roc a en el Valle de la Muerte, en California, se descubrieron dos almacenamientos que resultaron contener numerosos artefactos y alimentos perecederos. Adernas de restos de semillas de especies nativas, incluyendo mezquite y pinon, se encontraron dentro de los vestigios varias sernillas de cultivos introducidos, incluyendo melon, calabaza y frijol. EIalmacenamiento que contenia el mayor ruimero de semillas domesticadas parece datar de finales del siglo diecinueve y/ 0 principios del siglo veinte, y representa la primera evidencia arqueologica reportada de horticultura shoshoni en el suroeste de la Gran Cuenca. RESUME.-Des reconnaissances archeologiques conduites dans deux abris rocheux de la Vallee de la Mort en Californie ont permis de mettre au jour deux structures d'entreposage contenant plusieurs objets et denrees perissables. -

Dictionary of Cultivated Plants and Their Regions of Diversity Second Edition Revised Of: A.C
Dictionary of cultivated plants and their regions of diversity Second edition revised of: A.C. Zeven and P.M. Zhukovsky, 1975, Dictionary of cultivated plants and their centres of diversity 'N -'\:K 1~ Li Dictionary of cultivated plants and their regions of diversity Excluding most ornamentals, forest trees and lower plants A.C. Zeven andJ.M.J, de Wet K pudoc Centre for Agricultural Publishing and Documentation Wageningen - 1982 ~T—^/-/- /+<>?- •/ CIP-GEGEVENS Zeven, A.C. Dictionary ofcultivate d plants andthei rregion so f diversity: excluding mostornamentals ,fores t treesan d lowerplant s/ A.C .Zeve n andJ.M.J ,d eWet .- Wageninge n : Pudoc. -11 1 Herz,uitg . van:Dictionar y of cultivatedplant s andthei r centreso fdiversit y /A.C .Zeve n andP.M . Zhukovsky, 1975.- Me t index,lit .opg . ISBN 90-220-0785-5 SISO63 2UD C63 3 Trefw.:plantenteelt . ISBN 90-220-0785-5 ©Centre forAgricultura l Publishing and Documentation, Wageningen,1982 . Nopar t of thisboo k mayb e reproduced andpublishe d in any form,b y print, photoprint,microfil m or any othermean swithou t written permission from thepublisher . Contents Preface 7 History of thewor k 8 Origins of agriculture anddomesticatio n ofplant s Cradles of agriculture and regions of diversity 21 1 Chinese-Japanese Region 32 2 Indochinese-IndonesianRegio n 48 3 Australian Region 65 4 Hindustani Region 70 5 Central AsianRegio n 81 6 NearEaster n Region 87 7 Mediterranean Region 103 8 African Region 121 9 European-Siberian Region 148 10 South American Region 164 11 CentralAmerica n andMexica n Region 185 12 NorthAmerica n Region 199 Specieswithou t an identified region 207 References 209 Indexo fbotanica l names 228 Preface The aimo f thiswor k ist ogiv e thereade r quick reference toth e regionso f diversity ofcultivate d plants.Fo r important crops,region so fdiversit y of related wild species areals opresented .Wil d species areofte nusefu l sources of genes to improve thevalu eo fcrops .