Optical Characterization of Alternaria Spp. Contaminated Wheat Grain and Its Influence in Early Broilers Nutrition on Oxidative Stress
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nature and Effect of Alternaria Spp. Complex from Wheat Grain on Germination and Disease Transmission
Pak. J. Bot., 45(5): 1817-1824, 2013. NATURE AND EFFECT OF ALTERNARIA SPP. COMPLEX FROM WHEAT GRAIN ON GERMINATION AND DISEASE TRANSMISSION ANALÍA E. PERELLÓ1,2* AND SILVINA LARRÁN1 1CIDEFI (Centro de Investigaciones de Fitopatología) y Cátedra de Fitopatología 2CONICET-Facultad de Ciencias Agrarias y Forestales de la Universidad Nacional de La Plata, Calle 60 y 119 (1900) La Plata, Buenos Aires, Argentina. *Corresponding author’s e-mail: anaperello2@ yahoo.com.ar Abstract Diseases caused by Alternaria sp. are among the most common diseases of crops throughout the world. Alternaria sp. is a common component of the flora of wheat seed. Although isolation of Alternaria sp. from wheat (Triticum aestivum) seed has been reported in Argentina, development of the Alternaria blight in plants from infected seeds has not been demonstrated experimentally. Seed transmission of strains belonging to Alternaria tenuissima, A. alternata, A. infectoria, A. triticina, A. chlamydospora and related genera like Embellisia and Ulocladium sp. on wheat were investigated in the Argentinean growing area, on wheat cultivars Klein Escorpión and Buck Poncho. A. tenuissima was the dominant fungus in black pointed kernels. Transmission of all 42 seed-borne members of Alternaria complex from seeds to seedlings artificially inoculated was detected by trays seedling symptoms test. Among the fungi tested most isolates of Alternaria, Embellisia sp. and Ulocladium sp. produced distinct seed rot and seedling infection symptoms. This confirmed the seed-borne nature of these fungi. In each wheat cultivar tested inoculated seeds appreciably reduced their germination. The emerging coleoptile is externally infected by hyphal growth from the infected pericarp. -
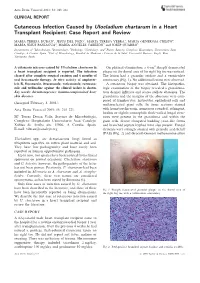
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Identification and Characterization of Alternaria Species Causing Leaf Spot
Eur J Plant Pathol (2017) 149:401–413 DOI 10.1007/s10658-017-1190-0 Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production Ilenia Siciliano & Giovanna Gilardi & Giuseppe Ortu & Ulrich Gisi & Maria Lodovica Gullino & Angelo Garibaldi Accepted: 22 February 2017 /Published online: 11 March 2017 # Koninklijke Nederlandse Planteziektenkundige Vereniging 2017 Abstract Alternaria species are common pathogens of Keywords Crucifers . Toxins . Leaf spot . Tenuazonic fruit and vegetables able to produce secondary metabo- acid lites potentially affecting human health. Twenty-nine isolates obtained from cabbage, cauliflower, wild and cultivated rocket were characterized and identified Introduction based on sporulation pattern and virulence; the phylo- β genetic analysis was based on the -tubulin gene. Most Alternaria species are saprophytes and ubiquitous Isolates were identified as A. alternata, A. tenuissima, in the environment, however some are plant pathogenic, A. arborescens, A. brassicicola and A. japonica. inducing diseases on a large variety of economically Pathogenicity was evaluated on plants under greenhouse important crops like cereals, oil-crops, vegetables and conditions. Two isolates showed low level of virulence fruits (Pitt and Hocking, 1997). Most Alternaria spp. on cultivated rocket while the other isolates showed produce chains of conidia with transverse and longitu- medium or high level of virulence. Isolates were also dinal septa with a tapering apical cell. Conidial size, characterized for their mycotoxin production on a mod- presence and size of a beak, the pattern of catenation ified Czapek-Dox medium. Production of the five and longitudinal and transverse septation are key taxo- Alternaria toxins, tenuazonic acid, alternariol, nomic features for this genus (Joly 1964; Ellis 1971 and alternariol monomethyl ether, altenuene and tentoxin 1976, Simmons 1992). -

Managing Alternaria Brown Spot by L.W
Managing Alternaria brown spot By L.W. Timmer, R. F. Reis, S.N. Mondal and N.A. Peres anker and greening may be problem for production of Fortune tan - the diseases that are on gerine in Spain. More recently, we Fig. 1. Alternaria brown spot lesions everyone’s minds, but we have confirmed the presence of the on a Minneola tangelo leaf. Note the still have to deal with the disease in Peru, where it is severe on characteristic necrosis running up Cmany everyday problems of produc - Minneolas, and in Iran, where it the veins. tion. Alternaria brown spot is the most causes problems mostly for Fortune for a few days on the grove floor, but serious disease of many popular tan - and Page production. then ceases as the leaves decay. Spores gerines and tangerine hybrids such as Tangerine cultivars, including Min - can also be produced on fruit and Minneola and Orlando tangelos, Mur - neolas, Orlandos, Novas, Lees, twigs, but are relatively few compared cotts, Sunburst, Novas and others. Ponkan, Murcotts, Dancy and many to the production on leaves. However, This disease probably requires more others, that are susceptible to ABS lesions on fruit and twigs may be im - intense management than any of the carry a gene for susceptibility to the portant in the overw-inter survival of other fungal diseases. toxin. That gene is dominant and all of the pathogen. Alternaria brown spot (ABS) is the offspring of susceptible parents are In the 2004-05 season, Alternaria caused by the fungus Alternaria alter - also susceptible. Many cultivars devel - pressure was very low and growers nata . -

Alternaria Infectoria Species-Group Associated with Black Point of Wheat in Argentina A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by SEDICI - Repositorio de la UNLP Blackwell Publishing Ltd Plant Pathology (2008) 57, 379 Doi: 10.1111/j.1365-3059.2007.01713.x Alternaria infectoria species-group associated with black point of wheat in Argentina A. Perellóa*, M. Morenoa and M. Sisternab aConsejo Nacional de Investigaciones Científicas y Tecnológicas; and bComisión de Investigaciones Científicas (Provincia de Buenos Aires) Centro de Investigaciones de Fitopatología, Facultad de Ciencias Agrarias y Forestales (Universidad Nacional de La Plata), 60 y 119, (1900) La Plata, Buenos Aires, Argentina Regional surveys are being conducted in Argentina to assess the presence studies have shown that grain samples are infected with A. alternata and of wheat (Triticum aestivum) pathogens on grains across the main A. infectoria species-groups ranging from low levels to 100%. cropping area. During 2001 and 2002, grain samples with a dark brown In Argentina, previous records of Alternaria spp. refer to A. alternata or blackish discoloration around the embryo end, known as black point, associated with black point in wheat. However, in this study the vast were observed on several cultivars across the wheat region of Buenos Aires majority of Alternaria strains conformed to the A. infectoria complex. The Province. incidence levels of this group are gaining importance and have increased Seed analysis by blotter and agar tests (Neergaard, 1979) showed up to in recent years probably due to changes in cropping systems in most of the 55% of prevalence (number of samples infected over the total) of Alternaria different agroclimatic zones of Argentina. -

The Medical Effects of Mold Exposure
AAAAI Position Statement February 2006 Environmental and occupational respiratory disorders Position paper The medical effects of mold exposure Robert K. Bush, MD, FAAAAI,a Jay M. Portnoy, MD, FAAAAI,b Andrew Saxon, MD, FAAAAI,c Abba I. Terr, MD, FAAAAI,d and Robert A. Wood, MDe Madison, Wis, Kansas City, Mo, Los Angeles and Palo Alto, Calif, and Baltimore, Md Exposure to molds can cause human disease through several well-defined mechanisms. In addition, many new mold-related Abbreviations used illnesses have been hypothesized in recent years that remain ABPA: Allergic bronchopulmonary aspergillosis largely or completely unproved. Concerns about mold exposure CRS: Chronic rhinosinusitis and its effects are so common that all health care providers, HP: Hypersensitivity pneumonitis particularly allergists and immunologists, are frequently faced MVOC: Volatile organic compound made by mold with issues regarding these real and asserted mold-related VOC: Volatile organic compound illnesses. The purpose of this position paper is to provide a state-of-the-art review of the role that molds are known to play in human disease, including asthma, allergic rhinitis, allergic bronchopulmonary aspergillosis, sinusitis, and hypersensitivity occupational respiratory pneumonitis. In addition, other purported mold-related pathology and are typically without specificity for the Environmental and illnesses and the data that currently exist to support them are involved fungus–fungal product purported to cause the carefully reviewed, as are the currently available approaches illness. disorders for the evaluation of both patients and the environment. In this position paper we will review the state of the (J Allergy Clin Immunol 2006;117:326-33.) science of mold-related diseases and provide interpre- Key words: Mold, fungi, hypersensitivity, allergy, asthma tation as to what is and what is not supported by scientific evidence. -

Alternaria Brassicicola – Brassicaceae Pathosystem: Insights Into the Infection Process and Resistance Mechanisms Under Optimized Artificial Bio-Assay
Eur J Plant Pathol https://doi.org/10.1007/s10658-018-1548-y Alternaria brassicicola – Brassicaceae pathosystem: insights into the infection process and resistance mechanisms under optimized artificial bio-assay Marzena Nowakowska & Małgorzata Wrzesińska & Piotr Kamiński & Wojciech Szczechura & Małgorzata Lichocka & Michał Tartanus & Elżbieta U. Kozik & Marcin Nowicki Accepted: 10 July 2018 # The Author(s) 2018 Abstract Heavy losses incited yearly by Alternaria that the plant genotype was crucial in determining its brassicicola on the vegetable Brassicaceae have response to the pathogen. The bio-assays for prompted our search for sources of genetic resistance A. brassicicola resistance were run under more stringent against the pathogen and the resultant disease, dark leaf lab conditions than the field tests (natural epidemics), spot. We optimized several parameters to test the perfor- resulting in identification of two interspecific hybrids mance of the plants under artificial inoculations with this that might be used in breeding programs. Based on the pathogen, including leaf age and position, inoculum results of the biochemical analyses, reactive oxygen concentration, and incubation temperature. Using these species and red-ox enzymes interplay has been suggested optimized conditions, we screened a collection of 38 to determine the outcome of the plant-A. brassicicola Brassicaceae cultigens with two methods (detached leaf interplay. Confocal microscopy analyses of the leaf sam- and seedlings). Our results show that either method can ples provided data on the pathogen mode of infection: be used for the A. brassicicola resistance breeding, and Direct epidermal infection or stomatal attack were relat- ed to plant resistance level against A. brassicicola among Electronic supplementary material The online version of this the cultigens tested. -

Foliar Diseases of Hydrangeas
Foliar Diseases of Hydrangeas Dr. Fulya Baysal-Gurel, Md Niamul Kabir and Adam Blalock Otis L. Floyd Nursery Research Center ANR-PATH-5-2016 College of Agriculture, Human and Natural Sciences Tennessee State University Hydrangeas are summer-flowering shrubs and are one of the showiest and most spectacular flowering woody plants in the landscape (Fig. 1). The appearance, health, and market value of hydrangea can be significantly influenced by the impact of different diseases. This publication focuses on common foliar diseases of hydrangea and their management recommendations. Powdery Mildew Fig 1. Hydrangea cv. Munchkin Causal agents: Golovinomyces orontii (formerly Erysiphe polygoni), Erysiphe poeltii, Microsphaera friesii, Oidium hortensiae Class: Leotiomycetes Powdery mildew pathogens have a very broad host range including hydrangeas. Some hydrangea species such as the bigleaf hydrangeas (Hydrangea macrophylla) are more susceptible to this disease while other species such as the oakleaf hydrangea (H. quercifolia), appear to be more resistant. In an outdoor environment, powdery mildew pathogens generally overwinter in the form of spores or fungal hyphae. In a heated greenhouse setting, powdery mildew can be active Fig 2. Powdery mildew year round. Spores and hyphae begin to grow when humidity is high but the leaf surface is dry. Warm days and cool nights also favor powdery mildew growth. The first sign of the disease is small fuzzy gray circles or patches on the upper surface of the leaf (Figs. 2 and 3). Inspecting these circular patches of fuzzy gray growth with a hand lens will reveal an intricate web of fungal hyphae. Sometimes small dark dots or structures can be seen within the web of fungal hyphae. -

Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit
Journal of Fungi Article Characterization of Alternaria Species Associated with Heart Rot of Pomegranate Fruit Francesco Aloi 1,2,†, Mario Riolo 1,3,4,† , Simona Marianna Sanzani 5, Annamaria Mincuzzi 6, Antonio Ippolito 6 , Ilenia Siciliano 7 , Antonella Pane 1,* , Maria Lodovica Gullino 7 and Santa Olga Cacciola 1,* 1 Department of Agriculture, Food and Environment, University of Catania, 95123 Catania, Italy; [email protected] (F.A.); [email protected] (M.R.) 2 Department of Agricultural, Food and Forest Sciences, University of Palermo, 90128 Palermo, Italy 3 Council for Agricultural Research and Agricultural Economy Analysis, Research Centre for Olive, Citrus and Tree Fruit–Rende CS (CREA- OFA), 87036 Rende, Italy 4 Department of Agricultural Science, Mediterranean University of Reggio Calabria, 89122 Reggio Calabria, Italy 5 CIHEAM Bari, Via Ceglie 9, 70010 Valenzano, Italy; [email protected] 6 Department of Soil, Plant, and Food Sciences, University of Bari Aldo Moro, Via Amendola 165/A, 70126 Bari, Italy; [email protected] (A.M.); [email protected] (A.I.) 7 Agroinnova—Centre of Competence for the Innovation in the Agro-Environmental Sector, University of Turin, 10095 Turin, Italy; [email protected] (I.S.); [email protected] (M.L.G.) * Correspondence: [email protected] (A.P.); [email protected] (S.O.C.) † These authors contributed equally to this work. Abstract: This study was aimed at identifying Alternaria species associated with heart rot disease of pomegranate fruit in southern Italy and characterizing their mycotoxigenic profile. A total of 42 Alternaria isolates were characterized. They were obtained from pomegranate fruits with symptoms Citation: Aloi, F.; Riolo, M.; Sanzani, of heart rot sampled in Apulia and Sicily and grouped into six distinct morphotypes based on S.M.; Mincuzzi, A.; Ippolito, A.; macro- and microscopic features. -

Fungi and Allergic Lower Respiratory Tract Diseases
Clinical reviews in allergy and immunology Series editors: Donald Y. M. Leung, MD, PhD, and Dennis K. Ledford, MD Fungi and allergic lower respiratory tract diseases Alan P. Knutsen, MD,a Robert K. Bush, MD,b Jeffrey G. Demain, MD,c David W. Denning, FRCP, FMedSci,d Anupma Dixit, PhD, MPH,e Abbie Fairs, MD,f Paul A. Greenberger, MD,g Barbara Kariuki, BS, MPH,a Hirohito Kita, MD,h Viswanath P. Kurup, PhD,i Richard B. Moss, MD,j Robert M. Niven, MD,d Catherine H. Pashley, MD,f Raymond G. Slavin, MD,e Hari M. Vijay, PhD, MPH,k and Andrew J. Wardlaw, MDf St Louis, Mo, Madison and Milwaukee, Wis, Anchorage, Alaska, Manchester and Leicester, United Kingdom, Chicago, Ill, Rochester, Minn, Palo Alto, Calif, and Ottawa, Ontario, Canada INFORMATION FOR CATEGORY 1 CME CREDIT Credit can now be obtained, free for a limited time, by reading the review PhD, Richard B. Moss, MD, Robert M. Niven, MD, Catherine H. Pashley, articles in this issue. Please note the following instructions. MD, Raymond G. Slavin, MD, Hari M. Vijay, PhD, MPH, and Andrew J. Method of Physician Participation in Learning Process: The core ma- Wardlaw, MD terial for these activities can be read in this issue of the Journal or online at Activity Objectives the JACI Web site: www.jacionline.org. The accompanying tests may only 1. To describe the most common environmental factors affecting fungal be submitted online at www.jacionline.org. Fax or other copies will not be spore dispersal . accepted. 2. To list the diagnostic criteria for allergic bronchopulmonary aspergil- Date of Original Release: February 2012. -

Multi-Locus Phylogeny of Pleosporales: a Taxonomic, Ecological and Evolutionary Re-Evaluation
available online at www.studiesinmycology.org StudieS in Mycology 64: 85–102. 2009. doi:10.3114/sim.2009.64.04 Multi-locus phylogeny of Pleosporales: a taxonomic, ecological and evolutionary re-evaluation Y. Zhang1, C.L. Schoch2, J. Fournier3, P.W. Crous4, J. de Gruyter4, 5, J.H.C. Woudenberg4, K. Hirayama6, K. Tanaka6, S.B. Pointing1, J.W. Spatafora7 and K.D. Hyde8, 9* 1Division of Microbiology, School of Biological Sciences, The University of Hong Kong, Pokfulam Road, Hong Kong SAR, P.R. China; 2National Center for Biotechnology Information, National Library of Medicine, National Institutes of Health, 45 Center Drive, MSC 6510, Bethesda, Maryland 20892-6510, U.S.A.; 3Las Muros, Rimont, Ariège, F 09420, France; 4CBS-KNAW Fungal Biodiversity Centre, P.O. Box 85167, 3508 AD, Utrecht, The Netherlands; 5Plant Protection Service, P.O. Box 9102, 6700 HC Wageningen, The Netherlands; 6Faculty of Agriculture & Life Sciences, Hirosaki University, Bunkyo-cho 3, Hirosaki, Aomori 036-8561, Japan; 7Department of Botany and Plant Pathology, Oregon State University, Corvallis, Oregon 93133, U.S.A.; 8School of Science, Mae Fah Luang University, Tasud, Muang, Chiang Rai 57100, Thailand; 9International Fungal Research & Development Centre, The Research Institute of Resource Insects, Chinese Academy of Forestry, Kunming, Yunnan, P.R. China 650034 *Correspondence: Kevin D. Hyde, [email protected] Abstract: Five loci, nucSSU, nucLSU rDNA, TEF1, RPB1 and RPB2, are used for analysing 129 pleosporalean taxa representing 59 genera and 15 families in the current classification ofPleosporales . The suborder Pleosporineae is emended to include four families, viz. Didymellaceae, Leptosphaeriaceae, Phaeosphaeriaceae and Pleosporaceae. In addition, two new families are introduced, i.e. -

The Arabidopsis Thaliana-Alternaria Brassicicola
The Arabidopsis thaliana-Alternaria brassicicola pathosystem: A model interaction for investigating seed transmission of necrotrophic fungi Stéphanie Pochon, Emmanuel Terrasson, Thomas Guillemette, Beatrice Iacomi-Vasilescu, Sonia Georgeault, Marjorie Juchaux, Romain Berruyer, Isabelle Debeaujon, Philippe Simoneau, Claire Campion To cite this version: Stéphanie Pochon, Emmanuel Terrasson, Thomas Guillemette, Beatrice Iacomi-Vasilescu, Sonia Georgeault, et al.. The Arabidopsis thaliana-Alternaria brassicicola pathosystem: A model inter- action for investigating seed transmission of necrotrophic fungi. Plant Methods, BioMed Central, 2012, 8, 10.1186/1746-4811-8-16. hal-01190780 HAL Id: hal-01190780 https://hal.archives-ouvertes.fr/hal-01190780 Submitted on 29 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License Pochon et al. Plant Methods 2012, 8:16 http://www.plantmethods.com/8/1/16 PLANT METHODS METHODOLOGY Open Access The Arabidopsis thaliana-Alternaria brassicicola pathosystem: A model interaction for investigating seed transmission of necrotrophic fungi Stephanie Pochon1,2,3, Emmanuel Terrasson1,2,3, Thomas Guillemette1,2,3, Beatrice Iacomi-Vasilescu4, Sonia Georgeault5, Marjorie Juchaux6, Romain Berruyer1,2,3, Isabelle Debeaujon7, Philippe Simoneau1,2,3* and Claire Campion1,2,3 Abstract Background: Seed transmission constitutes a major component of the parasitic cycle for several fungal pathogens.