The Arabidopsis Thaliana-Alternaria Brassicicola
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Alternaria Brassicicola)
Int.J.Curr.Microbiol.App.Sci (2020) 9(8): 2553-2559 International Journal of Current Microbiology and Applied Sciences ISSN: 2319-7706 Volume 9 Number 8 (2020) Journal homepage: http://www.ijcmas.com Original Research Article https://doi.org/10.20546/ijcmas.2020.908.292 In-vivo Management of Alternaria Leaf Spot of Cabbage (Alternaria brassicicola) Dwarkadas T. Bhere*, K. M. Solanke, Amrita Subhadarshini, Shashi Tiwari and Mohan K. Narode Department of Plant Pathology, Sam Higginbottom University of Agriculture, Technology and Sciences, Prayagraj, (U. P), India *Corresponding author ABSTRACT K e yw or ds An experiment was conducted for in-vivo management of Alternaria leaf spot of Cabbage. Alternaria leaf spot, The experiment was analyzed by using RBD (randomized block design) with three 2 Cabbage, replications in a plot size 2x2m . Eight treatments were taken i.e. Neem oil, Eucalyptus oil, Eucalyptus oil and Clove oil, Trichoderma viride, Neem oil + Trichoderma viride, Eucalyptus oil + Clove oil, Trichoderma viride, Clove oil + Trichoderma viride along with the control. Observations Trichoderma viride, were recorded at disease intensity 30, 45 and 60 (days after Transplanting), plant growth Neem oil parameters such a yield (q/ha). Experiment revealed that Neem oil significantly reduced the Alternaria leaf spot of Cabbage, where among the use Neem oil seedling treatment @ Article Info 5% increased the yield. The maximum cost benefit ratio was recorded by Neem oil (1:3.26) Thus according to experimental finding and results discussed in the earlier Accepted: chapter, it is concluded that Neem oil reduced the Alternaria leaf spot of Cabbage, where 22 July 2020 among the Neem oil seedling application found maximum yield was significantly superior Available Online: 10 August 2020 as compare to other treatments. -

Infection Cycle of Alternaria Brassicicola on Brassica Oleracea Leaves Under Growth Room Conditions
Plant Pathology (2018) 67, 1088–1096 Doi: 10.1111/ppa.12828 Infection cycle of Alternaria brassicicola on Brassica oleracea leaves under growth room conditions V. K. Macioszeka, C. B. Lawrenceb and A. K. Kononowicza* aDepartment of Genetics, Plant Molecular Biology and Biotechnology, Faculty of Biology and Environmental Protection, University of Lodz, 90-237 Lodz, Poland; and bDepartment of Biological Sciences, Virginia Tech, Blacksburg, VA 24061, USA Development of the necrotrophic fungus Alternaria brassicicola was evaluated during infection of three cabbage vari- eties: Brassica oleracea var. capitata f. alba ‘Stone Head’ (white cabbage), B. oleracea var. capitata f. rubra ‘Langedi- jker Dauer’ (red cabbage) and B. oleracea var. capitata f. sabauda ‘Langedijker Dauerwirsing’ (Savoy cabbage). Following inoculation of cabbage leaves, conidial germination, germ tube growth, and appressorium formation were analysed during the first 24 h of infection. Differences in the dynamics of fungal development on leaves were observed, e.g. approximately 40% of conidia germinated on Savoy cabbage leaves at 4 h post-inoculation (hpi) while only 20% germinated on red and white cabbage leaves. Leaf penetration on the three cabbage varieties mainly occurred through appressoria, rarely through stomata. Formation of infection cushions was found exclusively on red cabbage. Appresso- ria were first observed on red cabbage leaves at 6 hpi, and on white and Savoy cabbage leaves at 8 hpi. Conidiogenesis occurred directly from mature conidia at an early stage of fungal development (10 hpi), but later (48 hpi) it occurred through conidiophores. Disease progress and changes in the morphology of leaf surfaces were also observed. At the final 120 hpi measurement point, necroses on all investigated varieties were approximately the same size. -

Nature and Effect of Alternaria Spp. Complex from Wheat Grain on Germination and Disease Transmission
Pak. J. Bot., 45(5): 1817-1824, 2013. NATURE AND EFFECT OF ALTERNARIA SPP. COMPLEX FROM WHEAT GRAIN ON GERMINATION AND DISEASE TRANSMISSION ANALÍA E. PERELLÓ1,2* AND SILVINA LARRÁN1 1CIDEFI (Centro de Investigaciones de Fitopatología) y Cátedra de Fitopatología 2CONICET-Facultad de Ciencias Agrarias y Forestales de la Universidad Nacional de La Plata, Calle 60 y 119 (1900) La Plata, Buenos Aires, Argentina. *Corresponding author’s e-mail: anaperello2@ yahoo.com.ar Abstract Diseases caused by Alternaria sp. are among the most common diseases of crops throughout the world. Alternaria sp. is a common component of the flora of wheat seed. Although isolation of Alternaria sp. from wheat (Triticum aestivum) seed has been reported in Argentina, development of the Alternaria blight in plants from infected seeds has not been demonstrated experimentally. Seed transmission of strains belonging to Alternaria tenuissima, A. alternata, A. infectoria, A. triticina, A. chlamydospora and related genera like Embellisia and Ulocladium sp. on wheat were investigated in the Argentinean growing area, on wheat cultivars Klein Escorpión and Buck Poncho. A. tenuissima was the dominant fungus in black pointed kernels. Transmission of all 42 seed-borne members of Alternaria complex from seeds to seedlings artificially inoculated was detected by trays seedling symptoms test. Among the fungi tested most isolates of Alternaria, Embellisia sp. and Ulocladium sp. produced distinct seed rot and seedling infection symptoms. This confirmed the seed-borne nature of these fungi. In each wheat cultivar tested inoculated seeds appreciably reduced their germination. The emerging coleoptile is externally infected by hyphal growth from the infected pericarp. -

A Novel Arabidopsis Pathosystem Reveals Cooperation of Multiple
www.nature.com/scientificreports OPEN A novel Arabidopsis pathosystem reveals cooperation of multiple hormonal response-pathways in host resistance against the global crop destroyer Macrophomina phaseolina Mercedes M. Schroeder1, Yan Lai1,2, Miwa Shirai1, Natalie Alsalek1,3, Tokuji Tsuchiya 4, Philip Roberts5 & Thomas Eulgem 1* Dubbed as a “global destroyer of crops”, the soil-borne fungus Macrophomina phaseolina (Mp) infects more than 500 plant species including many economically important cash crops. Host defenses against infection by this pathogen are poorly understood. We established interactions between Mp and Arabidopsis thaliana (Arabidopsis) as a model system to quantitatively assess host factors afecting the outcome of Mp infections. Using agar plate-based infection assays with diferent Arabidopsis genotypes, we found signaling mechanisms dependent on the plant hormones ethylene, jasmonic acid and salicylic acid to control host defense against this pathogen. By profling host transcripts in Mp-infected roots of the wild-type Arabidopsis accession Col-0 and ein2/jar1, an ethylene/jasmonic acid-signaling defcient mutant that exhibits enhanced susceptibility to this pathogen, we identifed hundreds of genes potentially contributing to a diverse array of defense responses, which seem coordinated by complex interplay between multiple hormonal response-pathways. Our results establish Mp/Arabidopsis interactions as a useful model pathosystem, allowing for application of the vast genomics-related resources of this versatile model plant to the systematic investigation of previously understudied host defenses against a major crop plant pathogen. Te broad host-spectrum pathogen Macrophomina phaseolina (Mp) is a devastating soil-borne fungus that infects more than 500 plant species1–5. Many of these hosts are economically important crop plants including maize, soybean, canola, cotton, peanut, sunfower and sugar cane5–10. -

The Effects of Pathogen Infection on Nitrogen Remobilization in Arabidopsis Thaliana
University of Tennessee, Knoxville TRACE: Tennessee Research and Creative Exchange Masters Theses Graduate School 12-2006 The Effects of Pathogen Infection on Nitrogen Remobilization in Arabidopsis thaliana Michelle Ann Boercker University of Tennessee - Knoxville Follow this and additional works at: https://trace.tennessee.edu/utk_gradthes Part of the Ecology and Evolutionary Biology Commons Recommended Citation Boercker, Michelle Ann, "The Effects of Pathogen Infection on Nitrogen Remobilization in Arabidopsis thaliana. " Master's Thesis, University of Tennessee, 2006. https://trace.tennessee.edu/utk_gradthes/1506 This Thesis is brought to you for free and open access by the Graduate School at TRACE: Tennessee Research and Creative Exchange. It has been accepted for inclusion in Masters Theses by an authorized administrator of TRACE: Tennessee Research and Creative Exchange. For more information, please contact [email protected]. To the Graduate Council: I am submitting herewith a thesis written by Michelle Ann Boercker entitled "The Effects of Pathogen Infection on Nitrogen Remobilization in Arabidopsis thaliana." I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the equirr ements for the degree of Master of Science, with a major in Ecology and Evolutionary Biology. James Fordyce, Major Professor We have read this thesis and recommend its acceptance: Nathan Sanders, Joe Williams Accepted for the Council: Carolyn R. Hodges Vice Provost and Dean of the Graduate School (Original signatures are on file with official studentecor r ds.) To the Graduate Council: I am submitting herewith a thesis written by Michelle Ann Boercker entitled “The Effects of Pathogen Infection on Nitrogen Remobilization in Arabidopsis thaliana.” I have examined the final electronic copy of this thesis for form and content and recommend that it be accepted in partial fulfillment of the requirement for the degree of Master of Science, with a major in Ecology and Evolutionary Biology. -

Rice Hoja Blanca: a Complex Plant–Virus–Vector Pathosystem F.J
CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 2010 5, No. 043 Review Rice hoja blanca: a complex plant–virus–vector pathosystem F.J. Morales* and P.R. Jennings Address: International Centre for Tropical Agriculture, AA 6713, Cali, Colombia. *Correspondence: F.J. Morales. E-mail: [email protected] Received: 30 April 2010 Accepted: 24 June 2010 doi: 10.1079/PAVSNNR20105043 The electronic version of this article is the definitive one. It is located here: http://www.cabi.org/cabreviews g CAB International 2009 (Online ISSN 1749-8848) Abstract Rice hoja blanca (RHB; white leaf) devastated rice (Oryza sativa) plantings in tropical America for half a century, before scientists could either identify its causal agent or understand the nature of its cyclical epidemics. The association of the planthopper Tagosodes orizicolus with RHB outbreaks, 20 years after its emergence in South America, suggested the existence of a viral pathogen. However, T. orizicolus could also cause severe direct feeding damage (hopperburn) to rice in the absence of hoja blanca, and breeders promptly realized that the genetic basis of resistance to these problems was different. Furthermore, it was observed that the causal agent of RHB could only be trans- mitted by a relatively low proportion of the individuals in any given population of T. orizicolus and that the pathogen was transovarially transmitted to the progeny of the planthopper vectors, affecting their normal biology. An international rice germplasm screening effort was initiated in the late 1950s to identify sources of resistance against RHB and the direct feeding damage caused by T. -
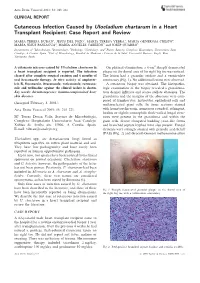
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Identification and Characterization of Alternaria Species Causing Leaf Spot
Eur J Plant Pathol (2017) 149:401–413 DOI 10.1007/s10658-017-1190-0 Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production Ilenia Siciliano & Giovanna Gilardi & Giuseppe Ortu & Ulrich Gisi & Maria Lodovica Gullino & Angelo Garibaldi Accepted: 22 February 2017 /Published online: 11 March 2017 # Koninklijke Nederlandse Planteziektenkundige Vereniging 2017 Abstract Alternaria species are common pathogens of Keywords Crucifers . Toxins . Leaf spot . Tenuazonic fruit and vegetables able to produce secondary metabo- acid lites potentially affecting human health. Twenty-nine isolates obtained from cabbage, cauliflower, wild and cultivated rocket were characterized and identified Introduction based on sporulation pattern and virulence; the phylo- β genetic analysis was based on the -tubulin gene. Most Alternaria species are saprophytes and ubiquitous Isolates were identified as A. alternata, A. tenuissima, in the environment, however some are plant pathogenic, A. arborescens, A. brassicicola and A. japonica. inducing diseases on a large variety of economically Pathogenicity was evaluated on plants under greenhouse important crops like cereals, oil-crops, vegetables and conditions. Two isolates showed low level of virulence fruits (Pitt and Hocking, 1997). Most Alternaria spp. on cultivated rocket while the other isolates showed produce chains of conidia with transverse and longitu- medium or high level of virulence. Isolates were also dinal septa with a tapering apical cell. Conidial size, characterized for their mycotoxin production on a mod- presence and size of a beak, the pattern of catenation ified Czapek-Dox medium. Production of the five and longitudinal and transverse septation are key taxo- Alternaria toxins, tenuazonic acid, alternariol, nomic features for this genus (Joly 1964; Ellis 1971 and alternariol monomethyl ether, altenuene and tentoxin 1976, Simmons 1992). -

Managing Alternaria Brown Spot by L.W
Managing Alternaria brown spot By L.W. Timmer, R. F. Reis, S.N. Mondal and N.A. Peres anker and greening may be problem for production of Fortune tan - the diseases that are on gerine in Spain. More recently, we Fig. 1. Alternaria brown spot lesions everyone’s minds, but we have confirmed the presence of the on a Minneola tangelo leaf. Note the still have to deal with the disease in Peru, where it is severe on characteristic necrosis running up Cmany everyday problems of produc - Minneolas, and in Iran, where it the veins. tion. Alternaria brown spot is the most causes problems mostly for Fortune for a few days on the grove floor, but serious disease of many popular tan - and Page production. then ceases as the leaves decay. Spores gerines and tangerine hybrids such as Tangerine cultivars, including Min - can also be produced on fruit and Minneola and Orlando tangelos, Mur - neolas, Orlandos, Novas, Lees, twigs, but are relatively few compared cotts, Sunburst, Novas and others. Ponkan, Murcotts, Dancy and many to the production on leaves. However, This disease probably requires more others, that are susceptible to ABS lesions on fruit and twigs may be im - intense management than any of the carry a gene for susceptibility to the portant in the overw-inter survival of other fungal diseases. toxin. That gene is dominant and all of the pathogen. Alternaria brown spot (ABS) is the offspring of susceptible parents are In the 2004-05 season, Alternaria caused by the fungus Alternaria alter - also susceptible. Many cultivars devel - pressure was very low and growers nata . -

Breeding Poplars for Disease Resistance
Breeding poplars , ' for 56 disease resistance . , ," . { " , .' ,./' -. .~. , FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Breeding poplars for disease resistance The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organilation of the United Nations concerning the legal status of any country. territory. city or area or of its authorities. or concerning the delimitation of its frontiers or boundaries. M-32 ISBN 92-5-102214-3 All rights reserved. No part of this publication may be reproduced. stored in a retrieval system. or transmitted in any form or by any means, electronic. mechanical, photocopying or otherwise, without the prior permission of the copyright ·owner. Applications for such permission, with a statement of the purpose and extent of the reproduction, should be addressed to the Director, Publications Division, Food and Agriculture Organization of the United Nations, Via delle Terme di Caracalla, 00100 Rome, Italy. - iii - FOREWORD Poplars are amongst the oldest contemporary angiosperm genera with over 125 species recorded as fossils and 30 to 40 species ~xisting today.They are dioecious and are more frequently propagated from cuttings than seed, Lending themseLves to the creation of cLones and the cuLtivation of recognizabLe and cLonally reproducible hybrids. They have Long been cuLtivated in Asia as welL as in Europe but their more recent deveLopment dates from the 18th century when North American poplars were imported to form hybrids with European species, a trend which is now extending to West and East Asia. Their utiLity lies in ~he frequently rapid growth potentiaL of species, their hybrids and certain clones as weLL as the wide use to which popLars can be put to provide industriaL wood, fodder for animaLs, sheLter, energy and timber for domestic and farm use. -

Concepts in Plant Disease Resistance
REVISÃO / REVIEW CONCEPTS IN PLANT DISEASE RESISTANCE FRANCISCO XAVIER RIBEIRO DO VALE1, J. E. PARLEVLIET2 & LAÉRCIO ZAMBOLIM1 1Departamento de Fitopatologia, Universidade Federal de Viçosa, CEP 36571-000, Viçosa, MG, Brasil, e-mail: [email protected]; 2Department of Plant Breeding (IVP), Wageningen Agricultural University, P.O. Box 386, 6700 AJ Wageningen, The Netherlands (Aceito para publicação em 13/08/2001) Autor para correspondência: Francisco Xavier Ribeiro do Vale RIBEIRO DO VALE, F.X., PARLEVLIET, J.E. & ZAMBOLIM, L. Concepts in plant disease resistance. Fitopatologia Brasileira 26:577- 589. 2001. ABSTRACT Resistance to nearly all pathogens occurs abundantly Race-specificity is not the cause of elusive resistance but the in our crops. Much of the resistance exploited by breeders is consequence of it. Understanding acquired resistance may of the major gene type. Polygenic resistance, although used open interesting approaches to control pathogens. This is even much less, is even more abundantly available. Many types of truer for molecular techniques, which already represent an resistance are highly elusive, the pathogen apparently adapting enourmously wide range of possibilities. Resistance obtained very easily them. Other types of resistance, the so-called through transformation is often of the quantitative type and durable resistance, remain effective much longer. The elusive may be durable in most cases. resistance is invariably of the monogenic type and usually of Key words: types of resistance; genetics of resistance; the hypersensitive type directed against specialised pathogens. acquired resistance. RESUMO Conceitos em resistência de plantas a doenças Na natureza a resistência à maioria das doenças ocorre tência durável. A resistência temporária é invariavelmente nas culturas. -

Systems Approaches in Epidemiology and Plant Disease Managetnent
Neth. J. Pl. Path. 99 (1993) Supplement 3: 161-171 Systems approaches in epidemiology and plant disease managetnent R. RABBINGE, W.A.H. ROSSING and W. VANDERWERF Department of Theoretical Production Ecology, Wageningen Agricultural University, P.O. Box 430, 6700 AK Wageningen, the Netherlands Accepted 28 October 1993 Abstract This contribution to Zadoks' liber amicorum reviews the developments in quantitative epidemio logy during the last decades. It elucidates the progress in this field and shows how empirical crop protection with many phenomenological aspects transformed into a science-based (inter)discipline. The availability of experimental tools and the rapid development and introduction of computers enabled the application of systems approaches which stimulated a revolution in thinking and caused · a considerable improvement of strategic and tactical decision making in crop protection. Zadoks played a crucial role in that development. Additional keyll'ords: simulation, modelling, crop protection Introduction Zadaks' contrilmtionto l'jJidemiology and plant disease management From the time of his arrival in Wageningen in 1956, Zadoks contributed to the systems approach in crop protection. As a fresh scientific worker in crop protection, he was aware of the necessity to determine fundamental epidemiological parameters such as infectious period, latency period and spore production under well-defined conditions, to better understand epidemiology of plant pathogenic fungi under field situations. When he started as a professor at the university, he received a so-called bride's contribution of one million Dutch guilders, an unbelievable amount nowadays, to construct climate cabinets and climate rooms that allowed him to undertake these studies. Zadoks' studies of funda mental epidemiological parameters laid the basis for his quantitative understanding of stripe rust epidemics in the field.