Nature and Effect of Alternaria Spp. Complex from Wheat Grain on Germination and Disease Transmission
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

TRITICALE Proceedings of an International Symposium El Batan, Mexico, 1-3 October 1973
ARCHIV IDRC-024e MACINT 11251 TRITICALE Proceedings of an international symposium El Batan, Mexico, 1-3 October 1973 Editors: Reginald Maclntyre/Marilyn Campbell IDRC-024e TRITICALE Proceedings of an international symposium, El Batan, Mexico, 1-3 October 1973* Editors: REGINALD MAcINTYRE/MARILYN CAMPBELL This symposium was co-sponsored by the Centro Internacional de Mejoramiento de Maiz y Trigo, the University of Manitoba, and theInternational Development Research Centre. Oi958 *The views expressed in this publication are those of the individualauthor(s) and do not necessarily represent the views of the International DevelopmentResearch Centre. ISBN 0-088936-028-6 UDC: 633.1 © 1974 International Development Research Centre Head Office: 60 Queen Street, Box 8500, Ottawa, CanadaK1G 3H9 Microfiche Edition $1 Contents Foreword W. David Hopper 5-7 List of Participants 8-11 Historical review of the development of triticale Arne Müntzing 13-30 Development of triticales in Western Europe E. Sanchez-Monge 31-39 Triticale-breeding experiments in Eastern Europe A. Kiss 41-50 Research work with 4x-Triticale in Germany (Berlin) K.-D.Krolow 51-60 Triticale research program in the United Kingdom R. S. Gregory 61-67 Progress in the development of triticale in Canada E. N. Larter 69-74 Triticale: its potential as a cereal crop in the United States of America R. I. Metzger 75-80 The triticale improvement program at CIMMYT F. J. Zillinsky 8 1-85 Prospects of triticale as a commercial crop in India J. P. Srivastava 87-92 Triticale breeding experiments in India N. S. Sisodia 93-101 Triticale research program in Iran M. -

Full Length Research Article DEVELOPMENT RESEARCH
Available online at http://www.journalijdr.com International Journal of DEVELOPMENT RESEARCH ISSN: 2230-9926 International Journal of Development Research Vol. 06, Issue, 12, pp.10765-10774, December, 2016 Full Length Research Article ECO-FRIENDLY MANAGEMENT OF STRIPE DISEASE OF BARLEY (HORDEUM VULGARE L.) BY PLANT EXTRACTS AND ANTAGONISTIC FUNGI *Mredula Trivedi and Archana Singh Department of Botany, Govt. M.S.J.P.G. College, Bharatpur, Rajasthan, India-321001 ARTICLE INFO ABSTRACT Article History: A search for an environmentally safe and economically viable strategy for control of plant Received 29th September, 2016 diseases has led to an increased use of plant based products in agriculture. To control the plant Received in revised form diseases, use of fungicides can impact the environment and human health. One method to 14th October, 2016 eliminate these drawbacks is promoting induced protection. This study investigated the use of Accepted 19th November, 2016 plants extracts and antagonistic fungi as a biological control of Drechslera graminea or as an th Published online 30 December, 2016 inducer of protection in barley plant against the pathogen and also evaluated the possible mechanisms. In the controlled laboratory conditions leaf extracts of Azadirachta indica, Ricinus Key Words: communis, Calotropis procera, Ocimum sanctum, Lawsonia rosea, Cassia tora and Barley, Drechslera Graminea, Crysanthemum indicum and native isolates of two fungal bioagents Trichoderma harzianum and Ecofriendly Management, T. viride were tested to examine their effectivity against D. graminea. Trichoderma sp. is an Plant Extract, ecofriendly organism that does not cause any harmful and side effect on human beings and Trichoderma harzianum and domestic animals when handled. -
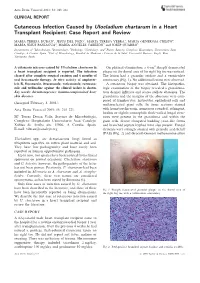
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

Identification and Characterization of Alternaria Species Causing Leaf Spot
Eur J Plant Pathol (2017) 149:401–413 DOI 10.1007/s10658-017-1190-0 Identification and characterization of Alternaria species causing leaf spot on cabbage, cauliflower, wild and cultivated rocket by using molecular and morphological features and mycotoxin production Ilenia Siciliano & Giovanna Gilardi & Giuseppe Ortu & Ulrich Gisi & Maria Lodovica Gullino & Angelo Garibaldi Accepted: 22 February 2017 /Published online: 11 March 2017 # Koninklijke Nederlandse Planteziektenkundige Vereniging 2017 Abstract Alternaria species are common pathogens of Keywords Crucifers . Toxins . Leaf spot . Tenuazonic fruit and vegetables able to produce secondary metabo- acid lites potentially affecting human health. Twenty-nine isolates obtained from cabbage, cauliflower, wild and cultivated rocket were characterized and identified Introduction based on sporulation pattern and virulence; the phylo- β genetic analysis was based on the -tubulin gene. Most Alternaria species are saprophytes and ubiquitous Isolates were identified as A. alternata, A. tenuissima, in the environment, however some are plant pathogenic, A. arborescens, A. brassicicola and A. japonica. inducing diseases on a large variety of economically Pathogenicity was evaluated on plants under greenhouse important crops like cereals, oil-crops, vegetables and conditions. Two isolates showed low level of virulence fruits (Pitt and Hocking, 1997). Most Alternaria spp. on cultivated rocket while the other isolates showed produce chains of conidia with transverse and longitu- medium or high level of virulence. Isolates were also dinal septa with a tapering apical cell. Conidial size, characterized for their mycotoxin production on a mod- presence and size of a beak, the pattern of catenation ified Czapek-Dox medium. Production of the five and longitudinal and transverse septation are key taxo- Alternaria toxins, tenuazonic acid, alternariol, nomic features for this genus (Joly 1964; Ellis 1971 and alternariol monomethyl ether, altenuene and tentoxin 1976, Simmons 1992). -

Alternaria Species and Mycotoxins Associated to Black Point of Cereals
Mycotoxins 63 (1), 39-46 (2013) 39 Proceedings Paper www.jstage.jst.go.jp/browse/myco Alternaria species and mycotoxins associated to black point of cereals * Maria T. AMATULLI, Francesca FANELLI, Antonio MORETTI, Giuseppina MULÈ, Antonio F. LOGRIECO Research National Council, Institute of Sciences of Food Production, Via Amendola 122/0 70126 Bari, Italy Key words : Alternaria; mycotoxins; wheat (Received January 25, 2013) Abstract Mycotoxins are secondary metabolites produced by several fungal species and represent a great concern for the economical and healthy implications on food and feed chain. Cereals are the primary source of human diet, wheat being the third most produced grain worldwide. Although Fusarium still represents the main source of mycotoxin contamination of wheat, in recent years, due also to evident climate changes that influence agricultural environment, other mycotoxingenic fungi have been pointed out as important wheat contaminants. Among these a disease called “Black Point”, caused by Alternaria spp., is increasing it importance as re-emerging risk. Diseases and mycotoxins (alternariol, altenuene, alternariol methyl-ether and tenuazolic acid) associated with Alternaria infection have been reported in several countries suggesting to deepen the knowledge about this genus. This paper summarizes the recent findings on wheat contamination by Alternaria spp and their related toxins. Introduction Alternaria spp. are worldwide distributed mainly as saprophyte in soil or in rot plant materials. They are also reported as contaminants of food commodities as well as fungal pathogens of several important crops, including cereals, oil crops, ornamentals, vegetable and fruits1, 2). Economic losses on fruits and vegetable trade are strongly dependent on the nature of the disease; they are usually lower compared to other fungal diseases but in some case they can lead to consistent losses3). -

Managing Alternaria Brown Spot by L.W
Managing Alternaria brown spot By L.W. Timmer, R. F. Reis, S.N. Mondal and N.A. Peres anker and greening may be problem for production of Fortune tan - the diseases that are on gerine in Spain. More recently, we Fig. 1. Alternaria brown spot lesions everyone’s minds, but we have confirmed the presence of the on a Minneola tangelo leaf. Note the still have to deal with the disease in Peru, where it is severe on characteristic necrosis running up Cmany everyday problems of produc - Minneolas, and in Iran, where it the veins. tion. Alternaria brown spot is the most causes problems mostly for Fortune for a few days on the grove floor, but serious disease of many popular tan - and Page production. then ceases as the leaves decay. Spores gerines and tangerine hybrids such as Tangerine cultivars, including Min - can also be produced on fruit and Minneola and Orlando tangelos, Mur - neolas, Orlandos, Novas, Lees, twigs, but are relatively few compared cotts, Sunburst, Novas and others. Ponkan, Murcotts, Dancy and many to the production on leaves. However, This disease probably requires more others, that are susceptible to ABS lesions on fruit and twigs may be im - intense management than any of the carry a gene for susceptibility to the portant in the overw-inter survival of other fungal diseases. toxin. That gene is dominant and all of the pathogen. Alternaria brown spot (ABS) is the offspring of susceptible parents are In the 2004-05 season, Alternaria caused by the fungus Alternaria alter - also susceptible. Many cultivars devel - pressure was very low and growers nata . -

Resistance in Spring Wheat to the Various Diseases Caused By
Resistance in spring wheat to the various diseases caused by Cochliobolus sativus by Aftabuddin Ahmed A thesis submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in Plant Pathology Montana State University © Copyright by Aftabuddin Ahmed (1989) Abstract: Sixteen spring wheat cultivate were tested for their resistance to the various diseases caused by Cochliobolus sativus (Ito and Kurib.) Drechsl. ex Dastur. Five Montana isolates and four Bangladesh isolates of the fungus were used in whole plant inoculations in the Plant Growth Center and in laboratory tests using detached leaves. Sources of resistance were detected and identified for different phases of the disease. Ten cultivars were resistant to root rot, eight cultivars were resistant to foliar spot blotch, and six cultivars were resistant to head blight or black point. A number of cultivars showed differential reaction to various phases of the disease, e.g., were resistant to root rot but were susceptible to foliar spot blotch. Six cultivars, namely Marberg, GP248, GP253, GP254 and GP255, were resistant to all phases of the disease. The isolates tested differed significantly in pathogenicity, but considerable shifting in ranking occurred between experiments. Isolates obtained from roots were able to attack foliage/heads and vice versa. The isolates from Bangladesh did not have a higher temperature requirement than the USA isolates. Some cultivars were resistant to all isolates from both Bangladesh and the USA. The maximum disease development for root rot, foliar and head blight/black point phases occurred at 30°C with a 72 hour exposure to moist conditions. The disease reactions on detached leaves were not consistent with those on intact leaves. -

Biological Control of Leaf Blight of Wheat Caused by Bipolaris Sorokinian M.M
Bull. Inst. Trop. Agr., Kyushu Univ. 39: 43-51, 2016 43 Biological control of leaf blight of wheat caused by Bipolaris sorokinian M.M. Hossain1)*, I. Hossain2) and K. M. Khalequzzaman3) Abstract Five different plant extracts including neem (Azadirachta indica), mehedi (Lawsonia alba), garlic clove (Allium sativum), rhizome of ginger (Zingiber officinales), seeds of black cumin (Nigella sativa), and BAU-Biofungicide (a Trichoderma based preparation) were used to evaluate the performance or effectiveness of those biological control agent on Bipolaris leaf blight of wheat and related pathogen (Bipolaris sorokiniana). Pathogenic reaction was observed in Bipolaris sorokiniana against different treatments by detached leaf method where leaf spot size was minimum (4.5mm) with BAU-Biofungicide and maximum leaf spot size (32.5mm) with control. Effect of seed treat- ment on wheat plant was evaluated by rolled paper towel method and BAU-Biofungicide, extracts of garlic clove and neem leaf at the value of 13%, 12% and 10%, respectively higher normal seedlings and BAU-Biofungicide also resulted 26.6% higher vigour index over control. Multiplication effect (seed treatment plus foliar spray of same treat- ment) of different treatments was examined to determine the efficacy for disease control. In pot and field experi- ments, though Bavistin and Tilt were most effective however, BAU-Biofungicide and extract of garlic clove were superior compared with the treatments used for controlling leaf blight of wheat. Multiplication effect of seed treat- ment plus foliar spray showed superior effect by BAU-Biofungicide including higher 1000-grain weight (43.92g) and grain yield (2.75 t/ha). Seed treatment with Bavistin and foliar spray with Tilt showed 1000-grain weight and grain yield by 47.12g and 3.0 t/ha, respectively. -

US EPA, Pesticide Product Label, Trivapro Fungicide,10/13/2020
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON, DC 20460 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION October 13, 2020 Adora Clark Fungicide Team Lead Syngenta Regulatory Affairs 410 Swing Rd. Greensboro, NC 27419-8300 Subject: Notification per PRN 98-10 – Addition of optional “Not for use in California” statements Product Name: Trivapro® Fungicide EPA Registration Number: 100-1613 Application Date: 8/24/2020 Decision Number: 566357 Dear Adora Clark: The Agency is in receipt of your Application for Pesticide Notification under Pesticide Registration Notice (PRN) 98-10 for the above referenced product. The Registration Division (RD) has conducted a review of this request for its applicability under PRN 98-10 and finds that the action requested falls within the scope of PRN 98-10. The label submitted with the application has been stamped “Notification” and will be placed in our records. Should you wish to add/retain a reference to the company’s website on your label, then please be aware that the website becomes labeling under the Federal Insecticide Fungicide and Rodenticide Act (FIFRA) and is subject to review by the Agency. If the website is false or misleading, the product would be misbranded and unlawful to sell or distribute under FIFRA section 12(a)(1)(E). 40 CFR 156.10(a)(5) list examples of statements EPA may consider false or misleading. In addition, regardless of whether a website is referenced on your product’s label, claims made on the website may not substantially differ from those claims approved through the registration process. Therefore, should the Agency find or if it is brought to our attention that a website contains false or misleading statements or claims substantially differing from the EPA approved registration, the website will be referred to the EPA’s Office of Enforcement and Compliance. -

Alternaria Infectoria Species-Group Associated with Black Point of Wheat in Argentina A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by SEDICI - Repositorio de la UNLP Blackwell Publishing Ltd Plant Pathology (2008) 57, 379 Doi: 10.1111/j.1365-3059.2007.01713.x Alternaria infectoria species-group associated with black point of wheat in Argentina A. Perellóa*, M. Morenoa and M. Sisternab aConsejo Nacional de Investigaciones Científicas y Tecnológicas; and bComisión de Investigaciones Científicas (Provincia de Buenos Aires) Centro de Investigaciones de Fitopatología, Facultad de Ciencias Agrarias y Forestales (Universidad Nacional de La Plata), 60 y 119, (1900) La Plata, Buenos Aires, Argentina Regional surveys are being conducted in Argentina to assess the presence studies have shown that grain samples are infected with A. alternata and of wheat (Triticum aestivum) pathogens on grains across the main A. infectoria species-groups ranging from low levels to 100%. cropping area. During 2001 and 2002, grain samples with a dark brown In Argentina, previous records of Alternaria spp. refer to A. alternata or blackish discoloration around the embryo end, known as black point, associated with black point in wheat. However, in this study the vast were observed on several cultivars across the wheat region of Buenos Aires majority of Alternaria strains conformed to the A. infectoria complex. The Province. incidence levels of this group are gaining importance and have increased Seed analysis by blotter and agar tests (Neergaard, 1979) showed up to in recent years probably due to changes in cropping systems in most of the 55% of prevalence (number of samples infected over the total) of Alternaria different agroclimatic zones of Argentina. -

INHERITANCE of ALTERNARIA RESISTANCE in WHEAT Division
INHERITANCE OF ALTERNARIA RESISTANCE IN WHEAT S. S. SOKHI*, L. M. JOSHI and M. V. RAO Division of Mycology and Plant Pathology, I.A.R.I., New Delhi (Accepted: 22-viii-73) LEAF blight of wheat has become of great concern in recent years in some parts of India as many of the varieties in cultivation are susceptible in one part or other of the country. The disease has become important, in recent years in Maharashtra, Bihar and West Bengal. The disease was first recorded in 1924 by Kulkarni from Bombay and by McRae from Bihar. A few subsequent reports on the disease appeared from U.P. (Mehta, 1960; Mathur, 1950), Orissa (Bose, 1956) and Andhra Pradesh (Rao, 1961). However, in all the above case the pathogenicity was not established by inoculation experiments and the causal organisms was not identified. Prasada and Prabhu (1962) identified the causal organism as a new species, Alternaria triticina. Some of the varieties such as Kenphad wheats and some Mexican wheats and their derivatives are susceptible to the disease. At present, the disease is ranked as one of the major diseases of wheat and causes substantial damage to the crop. The progress in respect of breeding varieties resistant to the disease would depend upon the knowledge of various sources of resistance and the mode of inheritance of genes for resistance. The present investigations report the results of such studies. www.IndianJournals.com Members Copy, Not for Commercial Sale MATERIALS AND METHODS Downloaded From IP - 61.247.228.217 on dated 27-Jun-2017 The mode of inheritance of jAltemaria blight resistance was studied in Fr and F2 of different crosses of 8 parents involving 7 resistant parents, viz., Agra Local, NP4 , NP53) NP809, NP824, E. -

Optical Characterization of Alternaria Spp. Contaminated Wheat Grain and Its Influence in Early Broilers Nutrition on Oxidative Stress
sustainability Article Optical Characterization of Alternaria spp. Contaminated Wheat Grain and Its Influence in Early Broilers Nutrition on Oxidative Stress Nikola Puvaˇca 1,* , Snežana Tanaskovi´c 2 , Vojislava Bursi´c 3, Aleksandra Petrovi´c 3, Jordan Merkuri 4, Tana Shtylla Kika 5, Dušan Marinkovi´c 3, Gorica Vukovi´c 6 and Magdalena Cara 4 1 Department of Engineering Management in Biotechnology, Faculty of Economics and Engineering Management in Novi Sad, University Business Academy in Novi Sad, Cve´carska2, 21000 Novi Sad, Serbia 2 Faculty of Agronomy in Caˇcak,Universityˇ of Kragujevac, Cara Dušana 34, 32102 Caˇcak,Serbia;ˇ [email protected] 3 Department for Phytomedicine and Environmental Protection, Faculty of Agriculture, University of Novi Sad, Trg Dositeja Obradovi´ca8, 21000 Novi Sad, Serbia; [email protected] (V.B.); [email protected] (A.P.); [email protected] (D.M.) 4 Department of Plant Protection, Faculty of Agriculture and Environment, Agricultural University of Tirana, Koder Kamez, 1029 Tirana, Albania; [email protected] (J.M.); [email protected] (M.C.) 5 Faculty of Veterinary Medicine, Agricultural University of Albania, Kodër Kamëz, SH1, 1000 Tirana, Albania; [email protected] 6 Faculty of Agriculture, University of Belgrade, Nemanjina 6, 11080 Belgrade-Zemun, Serbia; [email protected] * Correspondence: nikola.puvaca@fimek.edu.rs; Tel.: +381-65-219-1284 Citation: Puvaˇca,N.; Tanaskovi´c,S.; Abstract: The aim of this research was the visual characterization and investigating the effects of Bursi´c,V.; Petrovi´c,A.; Merkuri, J.; Alternaria spp. contaminated wheat grains in the starter stage of broilers nutrition on productive Shtylla Kika, T.; Marinkovi´c,D.; parameters and oxidative stress.