Identification and Characterization of Alternaria Species Causing Leaf Spot
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Nature and Effect of Alternaria Spp. Complex from Wheat Grain on Germination and Disease Transmission
Pak. J. Bot., 45(5): 1817-1824, 2013. NATURE AND EFFECT OF ALTERNARIA SPP. COMPLEX FROM WHEAT GRAIN ON GERMINATION AND DISEASE TRANSMISSION ANALÍA E. PERELLÓ1,2* AND SILVINA LARRÁN1 1CIDEFI (Centro de Investigaciones de Fitopatología) y Cátedra de Fitopatología 2CONICET-Facultad de Ciencias Agrarias y Forestales de la Universidad Nacional de La Plata, Calle 60 y 119 (1900) La Plata, Buenos Aires, Argentina. *Corresponding author’s e-mail: anaperello2@ yahoo.com.ar Abstract Diseases caused by Alternaria sp. are among the most common diseases of crops throughout the world. Alternaria sp. is a common component of the flora of wheat seed. Although isolation of Alternaria sp. from wheat (Triticum aestivum) seed has been reported in Argentina, development of the Alternaria blight in plants from infected seeds has not been demonstrated experimentally. Seed transmission of strains belonging to Alternaria tenuissima, A. alternata, A. infectoria, A. triticina, A. chlamydospora and related genera like Embellisia and Ulocladium sp. on wheat were investigated in the Argentinean growing area, on wheat cultivars Klein Escorpión and Buck Poncho. A. tenuissima was the dominant fungus in black pointed kernels. Transmission of all 42 seed-borne members of Alternaria complex from seeds to seedlings artificially inoculated was detected by trays seedling symptoms test. Among the fungi tested most isolates of Alternaria, Embellisia sp. and Ulocladium sp. produced distinct seed rot and seedling infection symptoms. This confirmed the seed-borne nature of these fungi. In each wheat cultivar tested inoculated seeds appreciably reduced their germination. The emerging coleoptile is externally infected by hyphal growth from the infected pericarp. -

In Vitro Evaluation of Plant Extracts and Bio- Agents Against Alternaria
International Journal of Chemical Studies 2018; 6(2): 504-507 P-ISSN: 2349–8528 E-ISSN: 2321–4902 IJCS 2018; 6(2): 504-507 In vitro evaluation of plant extracts and bio- © 2018 IJCS Received: 04-01-2018 agents against Alternaria tenuissima (Fr.) keissl Accepted: 05-02-2018 causing leaf blight of kodo millet K Hariprasad Department of Plant Pathology, UAS, GKVK, Bengaluru - 65 K Hariprasad, A Nagaraja, Suresh Patil and Rakesha *Project Coordinating Unit (Small Millets), ICAR, GKVK, Abstract Bengaluru, Karnataka, India Kodo millet (Paspalum scrobiculatum L.) is nutritionally important millet. Leaf blight has been a major production constraint and fungicidal sprays for the management of any disease on this crop may not be A Nagaraja economically viable and feasible as the farmers cultivating the crop are resource poor and the crop is less Department of Plant Pathology, remunerative, but is important in tribal and rainfed agriculture. Hence, botanicals and bio-agents were UAS, GKVK, Bengaluru - 65 *Project Coordinating Unit evaluated in vitro against Alternaria tenuissima the cause of leaf blight. Ten plant extracts and 14 bio- (Small Millets), ICAR, GKVK, agents were evaluated following dual culture. The results revealed 100 per cent inhibition of the mycelial Bengaluru, Karnataka, India growth of A. tenuissima by Eucalyptus sp. and Clerodendron infortunatum at 7.5 and 10.0 per cent concentrations. Among the fungal bio control agents tested, maximum inhibition (100 %) was recorded Suresh Patil in Trichoderma harzianum (NBAIR), followed by T. viride (81.38 %); whereas the bacterial bio agent Department of Plant Pathology, Bacillus amyloliquefaciens (P-42) showed only 77.40 % inhibition of mycelial growth revealing that UAS, GKVK, Bengaluru - 65 fungal antagonists were more effective than the bacterial antagonists. -

Genetic Variability in the Pistachio Late Blight Fungus, Alternaria Alternata
Mycol. Res. 105 (3): 300–306 (March 2001). Printed in the United Kingdom. 300 Genetic variability in the pistachio late blight fungus, Alternaria alternata Mallikarjuna K. ARADHYA*, Helen M. CHAN and Dan E. PARFITT Department of Pomology, University of California, One Shields Avenue, Davis, CA 95616, USA. E-mail: aradhya!ucdavis.edu Received 15 December 1999; accepted 21 August 2000 Genetic variation in the pistachio late blight fungus, Alternaria alternata, was investigated by restriction fragment length polymorphism (RFLP) in the rDNA region. Southern hybridization of EcoRI, HindIII, and XbaI digested fungal DNA with a RNA probe derived from Alt1, an rDNA clone isolated from a genomic library of the Japanese pear pathotype of A. alternata, revealed 34 different rDNA haplotypes among 56 isolates collected from four central valley locations in California. Analysis of molecular variation revealed a significant amount of genetic diversity within populations (85n8%), with only marginal variation accounting for differentiation among populations (14n2%, ΦST l 0n142). All isolates examined were highly pathogenic. The identity of the four geographic populations sampled was not evident in both cluster and principal component analyses, probably indicating either the selectively neutral nature of rDNA variation or prevalence of widespread gene flow among populations combined with uniform host-selection. INTRODUCTION 1998). Biochemical and molecular markers have been used to demonstrate the existence of multiple strains and to assess The genus Alternaria contains diverse and ubiquitous species of infraspecific variation in A. alternata at the protein and DNA fungi, including aggressive and opportunistic plant pathogens level (Petrunak & Christ 1992, Adachi et al. 1993, Weir et al. -
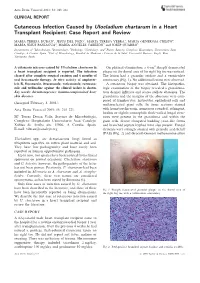
Cutaneous Infection Caused by Ulocladium Chartarum in a Heart Transplant Recipient: Case Report and Review
Acta Derm Venereol 2003; 83: 218–221 CLINICAL REPORT Cutaneous Infection Caused by Ulocladium chartarum in a Heart Transplant Recipient: Case Report and Review MARI´A TERESA DURA´ N1, JESU´ S DEL POZO2, MARI´A TERESA YEBRA3, MARI´A GENEROSA CRESPO4, MARI´A JESU´ S PANIAGUA4, MARI´A ANGELES CABEZO´ N5 and JOSEP GUARRO6 Departments of 1Microbiology, 2Dermatology, 3Pathology, 4Cardiology, and 5Plastic Surgery, Complexo Hospitalario Universitario Juan Canalejo, A Corun˜a, Spain, 6Unit of Microbiology, Facultat de Medicina i Cie`ncies de la Salut, Universitat Rovira i Virgili, Reus, Tarragona, Spain A cutaneous mycoses caused by Ulocladium chartarum in On physical examination, a 6-cm2 sharply demarcated a heart transplant recipient is reported. The infection plaque on the dorsal area of his right big toe was noticed. cleared after complete surgical excision and 6 months of The lesion had a granular surface and a vermiculate oral itraconazole therapy. In vitro activity of amphoter- consistency (Fig. 1). No additional lesions were observed. icin B, fluconazole, itraconazole, voriconazole, ravucona- A cutaneous biopsy was obtained. The histopatho- zole and terbinafine against the clinical isolate is shown. logic examination of the biopsy revealed a granuloma- Key words: dermatomycoses; immunocompromised host; tous dermal infiltrate and scarce stellate abscesses. The skin diseases. granuloma and the margins of the abscesses were com- posed of lymphocytes, histiocytes, epithelioid cells and (Accepted February 3, 2003.) multinucleated giant cells. In tissue sections stained Acta Derm Venereol 2003; 83: 218–221. with hematoxylin-eosin, numerous rounded, refringent, hyaline or slightly eosinophilic thick-walled fungal struc- Ma Teresa Dura´n Valle, Servicio de Microbiologı´a, tures were present in the granuloma and within the Complexo Hospitalario Universitario Juan Canalejo, giant cells. -

The Phylogeny of Plant and Animal Pathogens in the Ascomycota
Physiological and Molecular Plant Pathology (2001) 59, 165±187 doi:10.1006/pmpp.2001.0355, available online at http://www.idealibrary.com on MINI-REVIEW The phylogeny of plant and animal pathogens in the Ascomycota MARY L. BERBEE* Department of Botany, University of British Columbia, 6270 University Blvd, Vancouver, BC V6T 1Z4, Canada (Accepted for publication August 2001) What makes a fungus pathogenic? In this review, phylogenetic inference is used to speculate on the evolution of plant and animal pathogens in the fungal Phylum Ascomycota. A phylogeny is presented using 297 18S ribosomal DNA sequences from GenBank and it is shown that most known plant pathogens are concentrated in four classes in the Ascomycota. Animal pathogens are also concentrated, but in two ascomycete classes that contain few, if any, plant pathogens. Rather than appearing as a constant character of a class, the ability to cause disease in plants and animals was gained and lost repeatedly. The genes that code for some traits involved in pathogenicity or virulence have been cloned and characterized, and so the evolutionary relationships of a few of the genes for enzymes and toxins known to play roles in diseases were explored. In general, these genes are too narrowly distributed and too recent in origin to explain the broad patterns of origin of pathogens. Co-evolution could potentially be part of an explanation for phylogenetic patterns of pathogenesis. Robust phylogenies not only of the fungi, but also of host plants and animals are becoming available, allowing for critical analysis of the nature of co-evolutionary warfare. Host animals, particularly human hosts have had little obvious eect on fungal evolution and most cases of fungal disease in humans appear to represent an evolutionary dead end for the fungus. -

Enzymatic Effect of Different Alternaria Tenuissima Tic Effect of Different
Bulletin of Environment, Pharmacology and Life Sciences Bull. Env. Pharmacol. Life Sci., Vol 6 Special issue [2] 2017: 422-427 ©2017 Academy for Environment and Life Sciences, India Online ISSN 2277-1808 Journal’s URL:http://www.bepls.com CODEN: BEPLAD Global Impact Factor 0.533 Universal Impact Factor 0.9804 NAAS Rating 4.95 FULL LENGTH ARTICLE OPEN ACCESS Enzymatic Effect of Different Host Defense Induction against Alternaria tenuissima (Kunze ex pers.) Wiltshire causing Dieback Disease of Chilli C.S. Azad1*, A. Kumar1, G. Chand1 and R.D. Ranjan2 1Department of Plant Pathology, 2Department of Plant Breeding and Genetics, Bihar Agricultural University, Sabour, Bhagalpur-813210, India *Email: [email protected] ABSTRACT Dieback disease, caused by Alternaria tenuissima, (Kunze ex pers.) Wiltshire, is one of the most important disease that affecting all the plant parts of chilli. Among different methods of bioagent application, combination of both soil and root dip application was significantly superior over soil and root dip application. The accumulation of enzymes i.e. polyphenol oxidase (PPO), peroxidase (PO) and total phenol significantly increased upto 72 hours. Among bioagents highest significantly increase in PPO, PO and total phenol activity were recorded in Trichoderma harzianum PBAT-21 + Pseudomonas fluorescens PBAP-27 i.e. 0.034, 0.381 and 8.58 µmol/min/mg protein respectively. The no. of spot/leaf (2.33%), no. of infected leaves/plant (1.44%) and disease severity (13.33%) of dieback disease of chilli were minimum in combination of soil and root dip inoculation of bioagents Trichoderma harzianum PBAT-21 and Pseudomonas fluorescens PBAP-27 as compare to control. -

Isolation and Identification of Fungi from Leaves Infected with False Mildew on Safflower Crops in the Yaqui Valley, Mexico
Isolation and identification of fungi from leaves infected with false mildew on safflower crops in the Yaqui Valley, Mexico Eber Addi Quintana-Obregón 1, Maribel Plascencia-Jatomea 1, Armando Burgos-Hérnandez 1, Pedro Figueroa-Lopez 2, Mario Onofre Cortez-Rocha 1 1 Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Blvd. Luis Encinas y Rosales s/n, Colonia Centro. C.P. 83000 Hermosillo, Sonora, México. 2 Campo Experimental Norman E. Borlaug-INIFAP. C. Norman Borlaug Km.12 Cd. Obregón, Sonora C.P. 85000 3 1 0 2 Aislamiento e identificación de hongos de las hojas infectadas con la falsa cenicilla , 7 en cultivos de cártamo en el Valle del Yaqui, México 2 - 9 1 Resumen. La falsa cenicilla es una enfermedad que afecta seriamente los cultivos de cártamo en : 7 3 el Valle del Yaqui, México, y es causada por la infección de un hongo perteneciente al género A Ramularia. En el presente estudio, un hongo aislado de hojas contaminadas fue cultivado bajo Í G diferentes condiciones de crecimiento con la finalidad de estudiar su desarrollo micelial y O L producción de esporas, determinándose que el medio sólido de , 18 C de O Septoria tritici ° C I incubación y fotoperiodos de 12 h luz-oscuridad, fueron las condiciones más adecuadas para el M desarrollo del hongo. Este aislamiento fue identificado morfológicamente como Ramularia E D , pero genómicamente como , por lo que no se puede cercosporelloides Cercosporella acroptili A aún concluir que especie causa esta enfermedad. Adicionalmente, en la periferia de las N A C infecciones estudiadas se detectó la presencia de Alternaria tenuissima y Cladosporium I X cladosporioides. -

Managing Alternaria Brown Spot by L.W
Managing Alternaria brown spot By L.W. Timmer, R. F. Reis, S.N. Mondal and N.A. Peres anker and greening may be problem for production of Fortune tan - the diseases that are on gerine in Spain. More recently, we Fig. 1. Alternaria brown spot lesions everyone’s minds, but we have confirmed the presence of the on a Minneola tangelo leaf. Note the still have to deal with the disease in Peru, where it is severe on characteristic necrosis running up Cmany everyday problems of produc - Minneolas, and in Iran, where it the veins. tion. Alternaria brown spot is the most causes problems mostly for Fortune for a few days on the grove floor, but serious disease of many popular tan - and Page production. then ceases as the leaves decay. Spores gerines and tangerine hybrids such as Tangerine cultivars, including Min - can also be produced on fruit and Minneola and Orlando tangelos, Mur - neolas, Orlandos, Novas, Lees, twigs, but are relatively few compared cotts, Sunburst, Novas and others. Ponkan, Murcotts, Dancy and many to the production on leaves. However, This disease probably requires more others, that are susceptible to ABS lesions on fruit and twigs may be im - intense management than any of the carry a gene for susceptibility to the portant in the overw-inter survival of other fungal diseases. toxin. That gene is dominant and all of the pathogen. Alternaria brown spot (ABS) is the offspring of susceptible parents are In the 2004-05 season, Alternaria caused by the fungus Alternaria alter - also susceptible. Many cultivars devel - pressure was very low and growers nata . -

Estimated Burden of Fungal Infections in Namibia
Journal of Fungi Article Estimated Burden of Fungal Infections in Namibia Cara M. Dunaiski 1,* and David W. Denning 2 1 Department of Health and Applied Sciences, Namibia University of Science and Technology, 13 Jackson Kaujeua Street, Windhoek 9000, Namibia 2 National Aspergillosis Centre, Wythenshawe Hospital and the University of Manchester, Manchester M23 9LT, UK * Correspondence: [email protected]; Tel.: +264 61 207 2891 Received: 30 June 2019; Accepted: 13 August 2019; Published: 16 August 2019 Abstract: Namibia is a sub-Saharan country with one of the highest HIV infection rates in the world. Although care and support services are available that cater for opportunistic infections related to HIV, the main focus is narrow and predominantly aimed at tuberculosis. We aimed to estimate the burden of serious fungal infections in Namibia, currently unknown, based on the size of the population at risk and available epidemiological data. Data were obtained from the World Health Organization (WHO), Joint United Nations Programme on HIV/AIDS (UNAIDS), and published reports. When no data existed, risk populations were used to estimate the frequencies of fungal infections, using the previously described methodology. The population of Namibia in 2011 was estimated at 2,459,000 and 37% were children. Among approximately 516,390 adult women, recurrent vulvovaginal candidiasis ( 4 episodes /year) is estimated to occur in 37,390 (3003/100,000 females). Using a low international ≥ average rate of 5/100,000, we estimated 125 cases of candidemia, and 19 patients with intra-abdominal candidiasis. Among survivors of pulmonary tuberculosis (TB) in Namibia 2017, 112 new cases of chronic pulmonary aspergillosis (CPA) are likely, a prevalence of 354 post-TB and a total prevalence estimate of 453 CPA patients in all. -

Comparative Genomics of Alternaria Species Provides Insights Into the Pathogenic Lifestyle of Alternaria Brassicae – a Pathoge
Rajarammohan et al. BMC Genomics (2019) 20:1036 https://doi.org/10.1186/s12864-019-6414-6 RESEARCH ARTICLE Open Access Comparative genomics of Alternaria species provides insights into the pathogenic lifestyle of Alternaria brassicae – a pathogen of the Brassicaceae family Sivasubramanian Rajarammohan1,2, Kumar Paritosh3, Deepak Pental3 and Jagreet Kaur1* Abstract Background: Alternaria brassicae, a necrotrophic pathogen, causes Alternaria Leaf Spot, one of the economically important diseases of Brassica crops. Many other Alternaria spp. such as A. brassicicola and A. alternata are known to cause secondary infections in the A. brassicae-infected Brassicas. The genome architecture, pathogenicity factors, and determinants of host-specificity of A. brassicae are unknown. In this study, we annotated and characterised the recently announced genome assembly of A. brassicae and compared it with other Alternaria spp. to gain insights into its pathogenic lifestyle. Results: We also sequenced the genomes of two A. alternata isolates that were co-infecting B. juncea using Nanopore MinION sequencing for additional comparative analyses within the Alternaria genus. Genome alignments within the Alternaria spp. revealed high levels of synteny between most chromosomes with some intrachromosomal rearrangements. We show for the first time that the genome of A. brassicae, a large-spored Alternaria species, contains a dispensable chromosome. We identified 460 A. brassicae-specific genes, which included many secreted proteins and effectors. Furthermore, we have identified the gene clusters responsible for the production of Destruxin-B, a known pathogenicity factor of A. brassicae. Conclusion: The study provides a perspective into the unique and shared repertoire of genes within the Alternaria genus and identifies genes that could be contributing to the pathogenic lifestyle of A. -

Alternaria Infectoria Species-Group Associated with Black Point of Wheat in Argentina A
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by SEDICI - Repositorio de la UNLP Blackwell Publishing Ltd Plant Pathology (2008) 57, 379 Doi: 10.1111/j.1365-3059.2007.01713.x Alternaria infectoria species-group associated with black point of wheat in Argentina A. Perellóa*, M. Morenoa and M. Sisternab aConsejo Nacional de Investigaciones Científicas y Tecnológicas; and bComisión de Investigaciones Científicas (Provincia de Buenos Aires) Centro de Investigaciones de Fitopatología, Facultad de Ciencias Agrarias y Forestales (Universidad Nacional de La Plata), 60 y 119, (1900) La Plata, Buenos Aires, Argentina Regional surveys are being conducted in Argentina to assess the presence studies have shown that grain samples are infected with A. alternata and of wheat (Triticum aestivum) pathogens on grains across the main A. infectoria species-groups ranging from low levels to 100%. cropping area. During 2001 and 2002, grain samples with a dark brown In Argentina, previous records of Alternaria spp. refer to A. alternata or blackish discoloration around the embryo end, known as black point, associated with black point in wheat. However, in this study the vast were observed on several cultivars across the wheat region of Buenos Aires majority of Alternaria strains conformed to the A. infectoria complex. The Province. incidence levels of this group are gaining importance and have increased Seed analysis by blotter and agar tests (Neergaard, 1979) showed up to in recent years probably due to changes in cropping systems in most of the 55% of prevalence (number of samples infected over the total) of Alternaria different agroclimatic zones of Argentina. -

Optical Characterization of Alternaria Spp. Contaminated Wheat Grain and Its Influence in Early Broilers Nutrition on Oxidative Stress
sustainability Article Optical Characterization of Alternaria spp. Contaminated Wheat Grain and Its Influence in Early Broilers Nutrition on Oxidative Stress Nikola Puvaˇca 1,* , Snežana Tanaskovi´c 2 , Vojislava Bursi´c 3, Aleksandra Petrovi´c 3, Jordan Merkuri 4, Tana Shtylla Kika 5, Dušan Marinkovi´c 3, Gorica Vukovi´c 6 and Magdalena Cara 4 1 Department of Engineering Management in Biotechnology, Faculty of Economics and Engineering Management in Novi Sad, University Business Academy in Novi Sad, Cve´carska2, 21000 Novi Sad, Serbia 2 Faculty of Agronomy in Caˇcak,Universityˇ of Kragujevac, Cara Dušana 34, 32102 Caˇcak,Serbia;ˇ [email protected] 3 Department for Phytomedicine and Environmental Protection, Faculty of Agriculture, University of Novi Sad, Trg Dositeja Obradovi´ca8, 21000 Novi Sad, Serbia; [email protected] (V.B.); [email protected] (A.P.); [email protected] (D.M.) 4 Department of Plant Protection, Faculty of Agriculture and Environment, Agricultural University of Tirana, Koder Kamez, 1029 Tirana, Albania; [email protected] (J.M.); [email protected] (M.C.) 5 Faculty of Veterinary Medicine, Agricultural University of Albania, Kodër Kamëz, SH1, 1000 Tirana, Albania; [email protected] 6 Faculty of Agriculture, University of Belgrade, Nemanjina 6, 11080 Belgrade-Zemun, Serbia; [email protected] * Correspondence: nikola.puvaca@fimek.edu.rs; Tel.: +381-65-219-1284 Citation: Puvaˇca,N.; Tanaskovi´c,S.; Abstract: The aim of this research was the visual characterization and investigating the effects of Bursi´c,V.; Petrovi´c,A.; Merkuri, J.; Alternaria spp. contaminated wheat grains in the starter stage of broilers nutrition on productive Shtylla Kika, T.; Marinkovi´c,D.; parameters and oxidative stress.