ADVAIR HFA Safely and Effectively. See Full Prescribing Information for ADVAIR
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
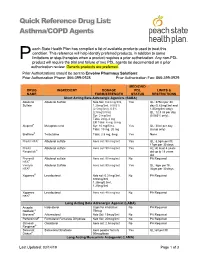
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

Utah Medicaid Pharmacy and Therapeutics Committee Drug
Utah Medicaid Pharmacy and Therapeutics Committee Drug Class Review Single Ingredient Nasal Corticosteroids Beclomethasone dipropionate (Qnasl) Beclomethasone dipropionate monohydrate (Beconase AQ) Budesonide (Rhinocort) Ciclesonide (Omnaris, Zetonna) Flunisolide (Generic) Fluticasone Furoate (Flonase Sensimist) Fluticasone Propionate (Flonase, Xhance) Mometasone Furoate (Nasonex) Triamcinolone Acetonide (Nasacort) AHFS Classification: 52.08.08 Corticosteroids (EENT) Final Report February 2018 Review prepared by: Valerie Gonzales, Pharm.D., Clinical Pharmacist Elena Martinez Alonso, B.Pharm., MSc MTSI, Medical Writer Vicki Frydrych, Pharm.D., Clinical Pharmacist Joanita Lake, B.Pharm., MSc EBHC (Oxon), Research Assistant Professor Joanne LaFleur, Pharm.D., MSPH, Associate Professor University of Utah College of Pharmacy University of Utah College of Pharmacy, Drug Regimen Review Center Copyright © 2018 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved Contents Abbreviations ................................................................................................................................................ 2 Executive Summary ....................................................................................................................................... 3 Introduction .................................................................................................................................................. 5 Table 1. Nasal corticosteroid products ............................................................................................. -

Drug Class Review Nasal Corticosteroids
Drug Class Review Nasal Corticosteroids Final Report Update 1 June 2008 The Agency for Healthcare Research and Quality has not yet seen or approved this report The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Dana Selover, MD Tracy Dana, MLS Colleen Smith, PharmD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Marian McDonagh, PharmD, Principal Investigator, Drug Effectiveness Review Project Copyright © 2008 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 1 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION ..........................................................................................................................5 Scope and Key Questions .......................................................................................................................7 METHODS ....................................................................................................................................9 Literature Search .....................................................................................................................................9 -

Formulary (List of Covered Drugs)
2018 Formulary (List of Covered Drugs) PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN Assurance Rx (HMO-POS) Essence Rx (HMO-POS) Esteem Rx (HMO-POS) Promise Rx (HMO-POS) Spirit Rx (HMO-POS) Surety Rx (HMO-POS) This formulary was updated on Nov. 21, 2018. For more recent information or other questions, please contact Security Health Plan Customer Service at 1-877-998-0998 or, for TTY users, 711, or visit https://www.securityhealth.org/medicareformulary. We are open 7 days a week, 8 a.m. to 8 p.m., from Oct. 1-March 31; and Monday through Friday, 8 a.m. to 8 p.m., from April 1-Sept. 30. Y0117_MC-778-0628-C-09-18 Effective date 12/1/2018 Updated 11/21/2018 Formulary ID 00018450, v.20 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us,” or “our,” it means Security Health Plan. When it refers to “plan” or “our plan,” it means Assurance Rx (HMO-POS), Essence Rx (HMO-POS), Promise Rx (HMO-POS), Spirit Rx (HMO- POS) or Surety Rx (HMO-POS). This document includes a list of the drugs (formulary) for our plan which is current as of Dec. 1, 2018. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. -

Aerospan (Flunisolide HFA, 80 Mcg) Inhalation Aerosol Use with AEROSPAN Canister Only for Oral Inhalation Only
AEROSPANTM (flunisolide HFA, 80 mcg) Inhalation Aerosol For Oral Inhalation Only Rx Only DESCRIPTION Flunisolide hemihydrate, the active component of AEROSPANTM (flunisolide HFA, 80 mcg) Inhalation Aerosol, is a corticosteroid having the chemical name 6α-Fluoro-11β, 16α, 17, 21 –tetrahydroxylpregna-1, 4-diene-3, 20-dione cyclic-16, 17-acetal with acetone, hemihydrate and the following chemical structure: O CH3 HO O C CH3 HO O . 1/2 H2O O F Flunisolide Hemihydrate Flunisolide hemihydrate is a white to creamy white crystalline powder with a molecular weight of 443.51 and an empirical formula of C24H31O6F •½ H2O. It is soluble in acetone, ethyl alcohol and HFA-134a and practically insoluble in water. AEROSPAN Inhalation Aerosol is a pressurized, metered-dose inhaler unit intended for oral inhalation only. The inhaler unit consists of a metal canister, a purple actuator, and a gray spacer. Each unit contains a 0.24 % w/w solution of flunisolide hemihydrate in 10:90 w/w ethanol:1,1,1,2-tetrafluoroethane (HFA 134a). After priming, each actuation delivers 139 mcg of flunisolide hemihydrate in 58 mg of solution from the canister valve and 80 mcg of flunisolide hemihydrate (equivalent to 78 mcg flunisolide) from the spacer at a flow rate of 30 L/min for 4 seconds. Using an in-vitro method at a fixed volume of 2 L, each actuation at the beginning of canister content delivers from the spacer 76 mcg (95% of the label claim) at a flow rate of 30 L/min, 61 mcg (76% of the label claim) at a flow rate of 20 L/min, 85 mcg (106% of the label claim) at a flow rate of 40 L/min, and 96 mcg (120% of the label claim) at a flow rate of 60 L/min. -
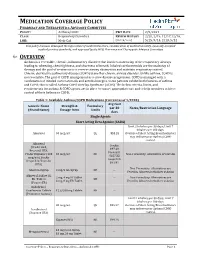
Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS)
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 2/9/2021 CLASS: Respiratory Disorders REVIEW HISTORY 2/20, 2/19, 12/17,12/16, LOB: Medi-Cal (MONTH/YEAR) 5/15, 9/14, 2/13, 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 1/2020) Avg Cost Generic Name Strength & Formulary per 30 Notes/Restriction Language (Brand Name) Dosage form Limits days Single Agents Short Acting Beta Agonist (SABA) Limit 2 inhalers per 30 days; Limit 7 inhalers per 180 days. Albuterol 90 mcg/act QL $53.28 Overuse of Short Acting Bronchodilators may indicate poor Asthma/COPD control. -

COPD/Asthma Class Update
Drug Use Research & Management Program Oregon State University, 500 Summer Street NE, E35, Salem, Oregon 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 © Copyright 2012 Oregon State University. All Rights Reserved Class Update: Asthma/COPD Medications Month/Year of Review: July 2014 Last Oregon Review: May 2012/November 2013 PDL Classes: Asthma Controller, Asthma Rescue, COPD Source Documents: OSU College of Pharmacy New drug(s): Anoro® Ellipta® (umeclidinium/vilanterol) Manufacturer: GSK/Theravance Dossier Received: Yes Current Status of PDL Classes: Chronic Obstructive Pulmonary Disease (COPD): Preferred Agents: IPRATROPIUM BROMIDE HFA AER AD, IPRATROPIUM BROMIDE SOLUTION, IPRATROPIUM/ALBUTEROL SULFATE AMPUL-NEB, TIOTROPIUM BROMIDE(SPIRIVA®) CAP W/DEV, IPRATROPIUM/ALBUTEROL (COMBIVENT®) RESPIMAT Non-Preferred Agents: AFORMOTEROL (BROVANA®), FORMOTEROL (PERFOROMIST), ROFLUMILAST (DALIRESP®), INDACATEROL (ARCAPTA®) NEOHALER, ACLIDINIUM (TUDORZA®) PRESSAIR, VILANTEROL/FLUTICASONE (BREO®) ELLIPTA Asthma Controllers and Asthma Rescue Preferred Agents: BECLOMETHASONE DIPROPIONATE(QVAR®), BUDESONIDE (PULMICORT FLEXHALER®), BUDESONIDE / FORMOTEROL FUMARATE (SYMBICORT®), FLUTICASONE PROPIONATE(FLOVENT HFA®), FLUTICASONE PROPIONATE(FLOVENT DISKUS®), FLUTICASONE/SALMETEROL(ADVAIR DISKUS®), FLUTICASONE/SALMETEROL(ADVAIR HFA®), FORMOTEROL (FORADIL®) AEROLIZER, MONTELUKAST SODIUM TAB CHEW/TABLET, SALMETEROL XINAFOATE (SEREVENT®), ALBUTEROL SULFATE SOLUTION/VIAL NEBS, PIRBUTEROL ACETATE, PROAIR® HFA, VENTOLIN® HFA Non-preferred Agents: CICLESONIDE -

Nasal Steroids C4730-A
Prior Authorization Criteria Nasal Steroids Policy Number: C4730-A CRITERIA EFFECTIVE DATES: ORIGINAL EFFECTIVE DATE LAST REVIEWED DATE NEXT REVIEW DATE 03/2012 7/29/2020 7/29/2021 LAST P&T J CODE TYPE OF CRITERIA APPROVAL/VERSION Q4 2020 3490 (NOC) RxPA 20201028C4730-A PRODUCTS AFFECTED: Flonase Sensimist (fluticasone furoate), Flunisolide, Qnasl (beclomethasone), Nasonex (mometasone), Omnaris (ciclesonide), Zetonna (ciclesonide), Beconase AQ (beclomethasone), Xhance (fluticasone propionate), Rhinocort (budesonide) DRUG CLASS: Nasal Steroids ROUTE OF ADMINISTRATION: Intranasal PLACE OF SERVICE: Retail Pharmacy The recommendation is that medications in this policy will be for pharmacy benefit coverage and member self-administered AVAILABLE DOSAGE FORMS: 24 HR NASAL SPR ALLERGY, ALLER-FLO SPR 50MCG, ALLERGY RELF SPR 50MCG, BECONASE AQ SPR 42MCG, BUDESONIDE SPR NASAL BUDESONIDE SPR NASALOTC, BUDESONIDE SUS 32MCG, CLARISPRAY SPR 50MCG FLONASE ALGY SPR 50MCG, FLONASE SENS SPR 27.5MCG, FLONASE-OTC SPR 50MCG FLONASE-RX SPR 50MCG120, FLUNISOLIDE SPR 0.025%, FLUTICASONE SPR 50MCG FLUTICASONE SPR 50MCGOTC, MOMETASONE SPR 50MCG, NASACORT ALR SPR 55MCG/AC, NASACORT ALR SPR 55MCGOTC, NASAL ALLGY SPR 55MCG/AC, NASALIDE INH SPR 0.025%, NASONEX SPR 50MCG, OMNARIS SPR NASAL, PROPEL IMP 370MCG, QNASL CHILD SPR 40MCG, QNASL SPR 80MCG, RA NASAL SPR ALLERGY, RHINOCORT SPR ALRGYOTC, RHINOCORT SPR AQUA, TRIAMCINOLON AER 55MCGOTC, VERAMYST NSL SPR 27.5MCG, XHANCE SPR 93MCG, ZETONNA AER 37MCG FDA-APPROVED USES: Allergic rhinitis; Seasonal and perennial, Non-allergic rhinitis XHANCE/NASONEX/RHINOCORT ONLY- nasal polyp treatment, QNASL/BECONASE AQ ONLY- prophylaxis of nasal polyp recurrence following surgical removal COMPENDIAL APPROVED OFF-LABELED USES: COVERAGE CRITERIA: INITIAL AUTHORIZATION DIAGNOSIS: Molina Healthcare, Inc. -
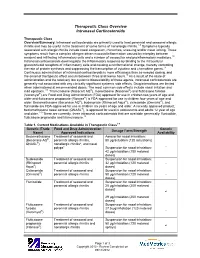
Therapeutic Class Overview Intranasal Corticosteroids
Therapeutic Class Overview Intranasal Corticosteroids Therapeutic Class Overview/Summary: Intranasal corticosteroids are primarily used to treat perennial and seasonal allergic rhinitis and may be useful in the treatment of some forms of nonallergic rhinitis.1-9 Symptoms typically associated with allergic rhinitis include nasal congestion, rhinorrhea, sneezing and/or nasal itching. These symptoms result from a complex allergen driven mucosal inflammation caused by interplay between resident and infiltrating inflammatory cells and a number of vasoactive and proinflammatory mediators.10 Intranasal corticosteroids downregulate the inflammatory response by binding to the intracellular glucocorticoid receptors of inflammatory cells and causing a conformational change, thereby controlling the rate of protein synthesis and suppressing the transcription of cytokine and chemokine genes.11 Continuous administration of intranasal corticosteroids is more efficacious than as-needed dosing, and the onset of therapeutic effect occurs between three and twelve hours.10 As a result of the route of administration and the relatively low systemic bioavailability of these agents, intranasal corticosteroids are generally not associated with any clinically significant systemic side effects. Drug interactions are limited when administered at recommended doses. The most common side effects include nasal irritation and mild epistaxis.1-9 Triamcinolone (Nasacort AQ®), mometasone (Nasonex®) and fluticasone furoate (Veramyst®) are Food and Drug Administration (FDA) approved for use in children two years of age and older and fluticasone propionate (Flonase®) is FDA-approved for use in children four years of age and older. Beclomethasone (Beconase AQ®), budesonide (Rhinocort Aqua®), ciclesonide (Omnaris®), and flunisolide are FDA-approved for use in children six years of age and older. -

Therapeutic Class Overview Intranasal Corticosteroids
Therapeutic Class Overview Intranasal Corticosteroids INTRODUCTION Intranasal corticosteroids are primarily used to treat perennial allergic rhinitis (PAR) and seasonal allergic rhinitis (SAR) and may be useful in the treatment of some forms of nonallergic rhinitis (Wallace et al, 2008). Symptoms associated with allergic rhinitis include nasal congestion, rhinorrhea, sneezing and/or nasal itching. These symptoms result from a complex allergen-driven mucosal inflammation caused by resident and infiltrating inflammatory cells and a number of vasoactive and proinflammatory mediators (Wallace et al, 2008). Treatment should consist of patient education, allergen avoidance activities and pharmacological therapies. Patients should be educated on how to avoid known triggers, such as aeroallergens, dust mites, molds and irritants whenever possible. In addition to environmental control measures, pharmacological therapies may be used to control symptoms. Intranasal corticosteroids down-regulate the inflammatory response by binding to the intracellular glucocorticoid receptors of inflammatory cells and causing a conformational change, thereby controlling the rate of protein synthesis and suppressing the transcription of cytokine and chemokine genes (Clinical Pharmacology®, 2017). Most intranasal corticosteroids are approved by the Food and Drug Administration (FDA) for the treatment of PAR and SAR. Mometasone (NASONEX®) carries an additional indication for the prophylaxis of SAR. NASACORT ALLERGY 24HR® (triamcinolone acetate), FLONASE® ALLERGY RELIEF (fluticasone propionate), FLONASE® SENSIMIST ALLERGY RELIEF (fluticasone furoate), and RHINOCORT® ALLERGY (budesonide) are all FDA-approved for over- the-counter use (Drugs@FDA, 2017). Nasal polyposis is an inflammatory condition of the nasal and sinus mucosa and usually presents as persistent nasal obstruction (Wallace et al, 200flu8). Two currently available intranasal corticosteroids, beclomethasone (BECONASE AQ®) and mometasone (NASONEX®) are also FDA-approved for the management of nasal polyps. -

Wo 2008/127291 A2
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date PCT (10) International Publication Number 23 October 2008 (23.10.2008) WO 2008/127291 A2 (51) International Patent Classification: Jeffrey, J. [US/US]; 106 Glenview Drive, Los Alamos, GOlN 33/53 (2006.01) GOlN 33/68 (2006.01) NM 87544 (US). HARRIS, Michael, N. [US/US]; 295 GOlN 21/76 (2006.01) GOlN 23/223 (2006.01) Kilby Avenue, Los Alamos, NM 87544 (US). BURRELL, Anthony, K. [NZ/US]; 2431 Canyon Glen, Los Alamos, (21) International Application Number: NM 87544 (US). PCT/US2007/021888 (74) Agents: COTTRELL, Bruce, H. et al.; Los Alamos (22) International Filing Date: 10 October 2007 (10.10.2007) National Laboratory, LGTP, MS A187, Los Alamos, NM 87545 (US). (25) Filing Language: English (81) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of national protection available): AE, AG, AL, AM, AT,AU, AZ, BA, BB, BG, BH, BR, BW, BY,BZ, CA, CH, (30) Priority Data: CN, CO, CR, CU, CZ, DE, DK, DM, DO, DZ, EC, EE, EG, 60/850,594 10 October 2006 (10.10.2006) US ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IS, JP, KE, KG, KM, KN, KP, KR, KZ, LA, LC, LK, (71) Applicants (for all designated States except US): LOS LR, LS, LT, LU, LY,MA, MD, ME, MG, MK, MN, MW, ALAMOS NATIONAL SECURITY,LLC [US/US]; Los MX, MY, MZ, NA, NG, NI, NO, NZ, OM, PG, PH, PL, Alamos National Laboratory, Lc/ip, Ms A187, Los Alamos, PT, RO, RS, RU, SC, SD, SE, SG, SK, SL, SM, SV, SY, NM 87545 (US). -

Steroid Nasal Sprays
Page 1 of 3 Steroid Nasal Sprays Steroid nasal sprays are medicines that are commonly used to treat allergies of the nose, such as hay fever and persistent rhinitis (inflammation of the nose). Steroid sprays reduce inflammation in the nose, and usually work well. Some people only need to use them for a few months of the year (hay fever). Other people may need to use them long-term (persistent rhinitis). You can buy some steroid nasal sprays from your supermarket or local pharmacy - for example, beclometasone and fluticasone. What are steroid nasal sprays? A steroid nasal spray is commonly used to treat allergies of the nose, such as hay fever and persistent rhinitis (inflammation of the nose). Steroid sprays reduce inflammation in the nose, and usually work well. There are a number of different steroid nasal sprays - these include: beclometasone, budesonide, flunisolide, fluticasone, mometasone and triamcinolone. They come in different brands. How to use a steroid nasal spray Blow your nose and shake the bottle. Tilt your head forward. Hold the spray bottle upright. Insert the tip of the spray bottle just inside one nostril. Close the other nostril with your other hand, and apply one or two sprays as prescribed. Breathe in as you spray (but do not sniff hard as the spray then travels past the nose to the throat). Do not angle the canister towards the middle or side of the nose, but straight up. With your head tilted forward, the spray should go to the back of your nose. Repeat in the other nostril. What if my nose is very blocked or runny? Sometimes a very blocked or runny nose will prevent the steroid spray from getting through to work.