Steroid Nasal Sprays
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
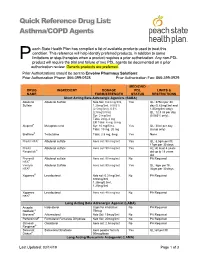
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Composition for Rectal Administration Combined with an Oral Alpha-Lipoic
(19) & (11) EP 2 162 125 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/136 (2006.01) A61K 31/01 (2006.01) 19.10.2011 Bulletin 2011/42 A61P 1/00 (2006.01) A61K 45/06 (2006.01) A61K 31/56 (2006.01) A61K 31/573 (2006.01) (2006.01) (21) Application number: 08768441.1 A61K 31/606 (22) Date of filing: 13.06.2008 (86) International application number: PCT/US2008/007401 (87) International publication number: WO 2008/156671 (24.12.2008 Gazette 2008/52) (54) Composition for rectal administration combined with an oral alpha-lipoic acid composition for the treatment of inflammatory bowel disease Zusammensetzung zur rektalen Verabreichung kombiniert mit einer oralen Zusammentzung enthaltend alpha Liponsäure zur Behandlung von entzündlicher Darmerkrankung Composition pour l’administration rectale en combinaison avec une composition orale de l’ acide alpha- lipoique pour le traitment des maladies intestinales inflammatoires (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-B1- 0 671 168 WO-A2-02/089796 HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT US-A- 4 657 900 US-A- 5 082 651 RO SE SI SK TR US-A- 5 378 470 (30) Priority: 13.06.2007 US 934505 P • MULDER CJ. ET AL.: ’Beclomethasone 06.02.2008 US 63745 P dipropionate (3mg) versus 5- aminosalicylic acid (2g) versus the combination of both (3mg/2g)as (43) Date of publication of application: retention enemas in active ulcerative proctitis’ 17.03.2010 Bulletin 2010/11 EUR.J.GASTROENTEROL HEPATOL vol. -

Inhaled Corticosteroid (ICS) Only – Metered Dose Inhalers (MDI)
Categorisation and Formulary Choices of Inhaled Corticosteroids for Adults Inhaled Corticosteroid (ICS) only – Metered Dose Inhalers (MDI) MDI Beclometasone dipropionate Use with a compatible Fluticasone Propionate spacer device Prescribe by BRAND Clenil Modulite® Qvar®(extrafine) Qvar®(extrafine) Flixotide® Evohaler MDI Easi-Breathe Low Dose 100mcg, two puffs twice a 50 mcg, two puffs twice a day 50 mcg, two puffs twice a day 50 mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) ≤400mcg BDP) £4.45/month £4.72/month £4.64/month £5.44/month Medium Dose 200mcg, two puffs twice a 100 mcg, two puffs twice a day 100 mcg, two puffs twice a day 125mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) 400-1000mcg BDP) £9.70/month £10.33/month £10.17/month £12.50/month High Dose 250mcg, two puffs twice a 100 mcg, four puffs twice a day 100 mcg, four puffs twice a day 250mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) >1000mcg BDP) £9.77/month £20.66/month £20.34/month £20.00/month Simultaneous specialist OR referral recommended 250mcg four puffs, twice a day (200 dose) £19.55/month Prices correct as per Drug Tariff and DM&D December2018 1st line choices (dark green, solid border) 2nd line choices (light green, dashed border) Approved by Hertfordshire Medicines Management Committee – October 2018 Inhaled Corticosteroid (ICS) only – Dry Powder Inhalers (DPI) Beclometasone DPI dipropionate Budesonide Fluticasone Propionate Prescribe -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

BSG Plan for IBD Patients During COVID19 Pandemic V1.5 349.88 KB
British Society of Gastroenterology (BSG) advice for management of inflammatory bowel diseases during the COVID-19 pandemic Date of publication: 22nd March 2020 Introduction Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is a condition in which the gastrointestinal immune system responds inappropriately. It is therefore often treated with immune suppression medications to control inflammation and to prevent ‘flares’, a worsening in symptoms, which may be unpredictable. It is known that 0.8% of people in the UK (approx. 524,000 patients) currently have IBD, but only 44% have been to a clinic in the last 3 years1,2. There will be many patients who will be worried about the effect of the Coronavirus pandemic (SARS-CoV-2 or COVID-19 disease) on their IBD and vice versa. During the COVID-19 outbreak we will do everything we can to keep our IBD patients safe. The biggest risks are related not only to the infection itself, but also the emergency reorganisation of hospital and general practice services to deal with the pandemic, meaning routine IBD services will be significantly affected. A combined approach covering both primary and secondary care is therefore required to keep vulnerable IBD patients out of hospital as much as possible. Insights from Hubei, China and from Italy suggest hospital admission for non-COVID-19 illness will provide a reservoir for further spread of infection. However, alterations to the way we deliver IBD care in the UK must be balanced against the risks of undertreated, active IBD. Importantly, patients with active IBD are likely to have a higher risk of infection both in the community and during inpatient care, even in the absence of immunosupressant treatment3. -

FF/UMEC/VI) Triple Therapy Versus Tiotropium Monotherapy in Patients with COPD
www.nature.com/npjpcrm ARTICLE OPEN Single-inhaler fluticasone furoate/umeclidinium/vilanterol (FF/UMEC/VI) triple therapy versus tiotropium monotherapy in patients with COPD Sandeep Bansal1, Martin Anderson2, Antonio Anzueto3,4, Nicola Brown5, Chris Compton6, Thomas C. Corbridge7,8, David Erb9, Catherine Harvey5, Morrys C. Kaisermann10, Mitchell Kaye11, David A. Lipson 10,12, Neil Martin6,13, Chang-Qing Zhu5 and ✉ Alberto Papi 14 Chronic obstructive pulmonary disease (COPD) treatment guidelines do not currently include recommendations for escalation directly from monotherapy to triple therapy. This 12-week, double-blind, double-dummy study randomized 800 symptomatic moderate-to-very-severe COPD patients receiving tiotropium (TIO) for ≥3 months to once-daily fluticasone furoate/umeclidinium/ vilanterol (FF/UMEC/VI) 100/62.5/25 mcg via ELLIPTA (n = 400) or TIO 18 mcg via HandiHaler (n = 400) plus matched placebo. Study endpoints included change from baseline in trough forced expiratory volume in 1 s (FEV1) at Days 85 (primary), 28 and 84 (secondary), health status (St George’s Respiratory Questionnaire [SGRQ] and COPD Assessment Test [CAT]) and safety. FF/UMEC/VI significantly improved trough FEV1 at all timepoints (Day 85 treatment difference [95% CI] 95 mL [62–128]; P < 0.001), and significantly improved SGRQ and CAT versus TIO. Treatment safety profiles were similar. Once-daily single-inhaler FF/UMEC/VI significantly improved lung function and health status versus once-daily TIO in symptomatic moderate-to-very-severe COPD 1234567890():,; patients, with a similar safety profile. npj Primary Care Respiratory Medicine (2021) 31:29 ; https://doi.org/10.1038/s41533-021-00241-z INTRODUCTION performed to date and a lack of updated recommendations Chronic obstructive pulmonary disease (COPD) is a major cause of based on the current body of evidence. -

A Practical Approach To, Diagnosis, Assessment and Management Of
REVIEW A practical approach to, diagnosis, Pract Neurol: first published as 10.1136/practneurol-2014-000821 on 8 May 2014. Downloaded from assessment and management of idiopathic intracranial hypertension Susan P Mollan,1 Keira A Markey,2 James D Benzimra,1 Andrew Jacks,1 Tim D Matthews,1 Michael A Burdon,1 Alex J Sinclair2,3 1Birmingham Neuro- ABSTRACT term to encompass primary raised intra- Ophthalmology Unit, Adult patients who present with papilloedema cranial pressure where there is no identi- Ophthalmology Department, — — University Hospitals Birmingham and symptoms of raised intracranial pressure fiable cause which we term IIH and 3 NHS Trust, Queen Elizabeth need urgent multidisciplinary assessment secondary causes of raised pressure. Hospital Birmingham, including neuroimaging, to exclude life- The diagnostic criteria of IIH are well Birmingham, UK 2 threatening causes. Where there is no apparent known and have evolved since Dandy’s Neurotrauma and 4 Neurodegeneration, School of underlying cause for the raised intracranial initial description in 1937 ; they include Clinical and Experimental pressure, patients are considered to have a CSF opening pressure of ≥25 cm H2O Medicine, College of Medical idiopathic intracranial hypertension (IIH). The (box 1).3 However, these criteria recom- and Dental Sciences, The incidence of IIH is increasing in line with the mend imaging only to exclude a venous Medical School, The University of Birmingham, Birmingham, UK global epidemic of obesity. There are sinus thrombosis in patients without the 3Department of Neurology, controversial issues in its diagnosis and typical IIH phenotype (obesity and University Hospital Birmingham management. This paper gives a practical female sex). We feel, however, that it is NHS Trust, Queen Elizabeth Hospital Birmingham, approach to assessing patients with essential to exclude venous sinus throm- Birmingham, UK papilloedema, its investigation and the bosis (using MRI or CT with venography) subsequent management of patients with IIH. -

Utah Medicaid Pharmacy and Therapeutics Committee Drug
Utah Medicaid Pharmacy and Therapeutics Committee Drug Class Review Single Ingredient Nasal Corticosteroids Beclomethasone dipropionate (Qnasl) Beclomethasone dipropionate monohydrate (Beconase AQ) Budesonide (Rhinocort) Ciclesonide (Omnaris, Zetonna) Flunisolide (Generic) Fluticasone Furoate (Flonase Sensimist) Fluticasone Propionate (Flonase, Xhance) Mometasone Furoate (Nasonex) Triamcinolone Acetonide (Nasacort) AHFS Classification: 52.08.08 Corticosteroids (EENT) Final Report February 2018 Review prepared by: Valerie Gonzales, Pharm.D., Clinical Pharmacist Elena Martinez Alonso, B.Pharm., MSc MTSI, Medical Writer Vicki Frydrych, Pharm.D., Clinical Pharmacist Joanita Lake, B.Pharm., MSc EBHC (Oxon), Research Assistant Professor Joanne LaFleur, Pharm.D., MSPH, Associate Professor University of Utah College of Pharmacy University of Utah College of Pharmacy, Drug Regimen Review Center Copyright © 2018 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved Contents Abbreviations ................................................................................................................................................ 2 Executive Summary ....................................................................................................................................... 3 Introduction .................................................................................................................................................. 5 Table 1. Nasal corticosteroid products ............................................................................................. -

Drug Class Review Nasal Corticosteroids
Drug Class Review Nasal Corticosteroids Final Report Update 1 June 2008 The Agency for Healthcare Research and Quality has not yet seen or approved this report The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Dana Selover, MD Tracy Dana, MLS Colleen Smith, PharmD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Marian McDonagh, PharmD, Principal Investigator, Drug Effectiveness Review Project Copyright © 2008 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 1 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION ..........................................................................................................................5 Scope and Key Questions .......................................................................................................................7 METHODS ....................................................................................................................................9 Literature Search .....................................................................................................................................9 -

Pharmacology/Therapeutics II Block III Lectures 2013-14
Pharmacology/Therapeutics II Block III Lectures 2013‐14 66. Hypothalamic/pituitary Hormones ‐ Rana 67. Estrogens and Progesterone I ‐ Rana 68. Estrogens and Progesterone II ‐ Rana 69. Androgens ‐ Rana 70. Thyroid/Anti‐Thyroid Drugs – Patel 71. Calcium Metabolism – Patel 72. Adrenocorticosterioids and Antagonists – Clipstone 73. Diabetes Drugs I – Clipstone 74. Diabetes Drugs II ‐ Clipstone Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones Date: Thursday, March 20, 2014-8:30 AM Reading Assignment: Katzung, Chapter 37 Key Concepts and Learning Objectives To review the physiology of neuroendocrine regulation To discuss the use neuroendocrine agents for the treatment of representative neuroendocrine disorders: growth hormone deficiency/excess, infertility, hyperprolactinemia Drugs discussed Growth Hormone Deficiency: . Recombinant hGH . Synthetic GHRH, Recombinant IGF-1 Growth Hormone Excess: . Somatostatin analogue . GH receptor antagonist . Dopamine receptor agonist Infertility and other endocrine related disorders: . Human menopausal and recombinant gonadotropins . GnRH agonists as activators . GnRH agonists as inhibitors . GnRH receptor antagonists Hyperprolactinemia: . Dopamine receptor agonists 1 Pharmacology & Therapeutics Neuroendocrine Pharmacology: Hypothalamic and Pituitary Hormones, March 20, 2014 Lecture Ajay Rana, Ph.D. 1. Overview of Neuroendocrine Systems The neuroendocrine