Utah Medicaid Pharmacy and Therapeutics Committee Drug
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
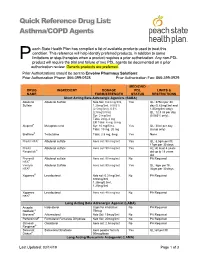
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

A Comparison of Fluticasone Propionate, 1 Mg Daily, with Beclomethasone Dipropionate, 2 Mg Daily, in the Treatment of Severe Asthma
Copyright ©ERS Joumals Ltd 1993 Eur Respir J , 1993, 6, Sn-884 European Respiratory Joumal Printed in UK - all rights reserved ISSN 0903 - 1936 A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma N.C. Bames*, G. Marone**, G.U. Di Maria***, S. Visser, I. Utama++, S.L. Payne+++, on behalf of an International Study Group A comparison of fluticasone propionate. 1 mg daily, with beclomethasone dipropionate, • The London Chest Hospital, London, 2 mg daily, in the treatment of severe asthma. N. C. Bames, G. Marone, G.U. Di Maria, UK. ** Servizio di Allergologia e S. Visser. l Utama, S.L Payne, on behalf of an International Study Group. @ERS Immunologia Clinica. I Clinica Medica Journals Ltd 1993. Universita, Napoli, Italy. *** lnstituto Malattie Respiratorie, Ospedale Tomaselli, ABSTRACT: We wanted w compare the efficacy and safety of fluticasone propi Catania, Sicily, Italy. onate, a new topically active inhaled corticosteroid, to that of high dose beclo + H.F. Verwoerd Hospital, Pretoria, South methasone dipropionate, in severe adult asthma. Africa. ++ St Laurentius Ziekenhius, 1 Patients currently receiving between 1.5-2.0 mg·day- of an inhaled corticoster CV Roermond, The Netherlands. +++ oid were treated for six weeks in a double-blind, randomized, parallel group study Glaxo Group Research Ltd, Greenford, with 1 mg·day-1 fluticasone propionate (n•82), or 2 mg·day·1 beclometbasone Middlesex, UK. dipropionate (n•72). Mean morning peak expirarory flow rates (PEFR) increased from 303 w 321 Correspondence: N.C. Bames l·min-1 with fluticasone propionate, and from 294 w 319 l·min·1 with beclometbasone The London Chest Hospital dipropionate. -

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Nitrate Prodrugs Able to Release Nitric Oxide in a Controlled and Selective
Europäisches Patentamt *EP001336602A1* (19) European Patent Office Office européen des brevets (11) EP 1 336 602 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.7: C07C 205/00, A61K 31/00 20.08.2003 Bulletin 2003/34 (21) Application number: 02425075.5 (22) Date of filing: 13.02.2002 (84) Designated Contracting States: (71) Applicant: Scaramuzzino, Giovanni AT BE CH CY DE DK ES FI FR GB GR IE IT LI LU 20052 Monza (Milano) (IT) MC NL PT SE TR Designated Extension States: (72) Inventor: Scaramuzzino, Giovanni AL LT LV MK RO SI 20052 Monza (Milano) (IT) (54) Nitrate prodrugs able to release nitric oxide in a controlled and selective way and their use for prevention and treatment of inflammatory, ischemic and proliferative diseases (57) New pharmaceutical compounds of general effects and for this reason they are useful for the prep- formula (I): F-(X)q where q is an integer from 1 to 5, pref- aration of medicines for prevention and treatment of in- erably 1; -F is chosen among drugs described in the text, flammatory, ischemic, degenerative and proliferative -X is chosen among 4 groups -M, -T, -V and -Y as de- diseases of musculoskeletal, tegumental, respiratory, scribed in the text. gastrointestinal, genito-urinary and central nervous sys- The compounds of general formula (I) are nitrate tems. prodrugs which can release nitric oxide in vivo in a con- trolled and selective way and without hypotensive side EP 1 336 602 A1 Printed by Jouve, 75001 PARIS (FR) EP 1 336 602 A1 Description [0001] The present invention relates to new nitrate prodrugs which can release nitric oxide in vivo in a controlled and selective way and without the side effects typical of nitrate vasodilators drugs. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Composition for Rectal Administration Combined with an Oral Alpha-Lipoic
(19) & (11) EP 2 162 125 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 31/136 (2006.01) A61K 31/01 (2006.01) 19.10.2011 Bulletin 2011/42 A61P 1/00 (2006.01) A61K 45/06 (2006.01) A61K 31/56 (2006.01) A61K 31/573 (2006.01) (2006.01) (21) Application number: 08768441.1 A61K 31/606 (22) Date of filing: 13.06.2008 (86) International application number: PCT/US2008/007401 (87) International publication number: WO 2008/156671 (24.12.2008 Gazette 2008/52) (54) Composition for rectal administration combined with an oral alpha-lipoic acid composition for the treatment of inflammatory bowel disease Zusammensetzung zur rektalen Verabreichung kombiniert mit einer oralen Zusammentzung enthaltend alpha Liponsäure zur Behandlung von entzündlicher Darmerkrankung Composition pour l’administration rectale en combinaison avec une composition orale de l’ acide alpha- lipoique pour le traitment des maladies intestinales inflammatoires (84) Designated Contracting States: (56) References cited: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR EP-B1- 0 671 168 WO-A2-02/089796 HR HU IE IS IT LI LT LU LV MC MT NL NO PL PT US-A- 4 657 900 US-A- 5 082 651 RO SE SI SK TR US-A- 5 378 470 (30) Priority: 13.06.2007 US 934505 P • MULDER CJ. ET AL.: ’Beclomethasone 06.02.2008 US 63745 P dipropionate (3mg) versus 5- aminosalicylic acid (2g) versus the combination of both (3mg/2g)as (43) Date of publication of application: retention enemas in active ulcerative proctitis’ 17.03.2010 Bulletin 2010/11 EUR.J.GASTROENTEROL HEPATOL vol. -

Flonase Sensimist Allergy Relief (Fluticasone Furoate)
Flonase® Sensimist™ Allergy Relief (fluticasone furoate) – Rx-to-OTC Approval • On February 8, 2017, GlaxoSmithKline Consumer Healthcare announced the launch of Flonase Sensimist Allergy Relief (fluticasone furoate) nasal spray, as an over the counter (OTC) treatment to temporarily relieve symptoms of hay fever or other upper respiratory allergies: nasal congestion; runny nose; sneezing; itchy nose; and itchy, watery eyes (in ages 12 years and older). — Flonase Sensimist Allergy Relief contains 27.5 mcg/spray of fluticasone furoate. — Flonase Sensimist Allergy Relief should not be used in children less than 2 years of age. • Previously, fluticasone furoate was only available by prescription as Veramyst®. Veramyst is no longer commercially available. • Fluticasone is also available OTC as brand (Flonase® Allergy Relief, Children’s Flonase® Allergy Relief) and generic products containing 50 mcg/spray of fluticasone propionate. — These products share the same indications as Flonase Sensimist Allergy Relief, but are not intended for children under 4 years of age. • Prescription fluticasone propionate nasal spray (50 mcg/spray) is available generically and indicated for the management of the nasal symptoms of perennial non-allergic rhinitis in adult and pediatric patients aged 4 years and older. • Warnings for Flonase Sensimist Allergy Relief state the following: — Do not use: in children under 2 years of age, to treat asthma, if there is an injury or surgery to the nose that is not fully healed, or if an allergic reaction to Flonase Sensimist Allergy Relief or any of its ingredients has occurred. — Ask a doctor prior to use if the patient has or had glaucoma or cataracts. -

Difference Between Patient-Reported Side Effects of Ciclesonide Versus fluticasone Propionate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2010) 104, 1825e1833 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Difference between patient-reported side effects of ciclesonide versus fluticasone propionate Thys van der Molen a, Juliet M. Foster a,b, Manfred Caeser c,e, Thomas Mu¨ller c, Dirkje S. Postma d,* a Department of General Practice, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands b Woolcock Institute of Medical Research, 431 Glebe Point Rd, Glebe NSW 2037, Sydney, Australia c Nycomed GmbH, Byk-Gulden-Straße 2, 78467 Konstanz, Konstanz, Germany d Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands Received 18 January 2010; accepted 26 May 2010 Available online 2 July 2010 KEYWORDS Summary Adverse events; Rationale: Patient-reported outcomes provide new insights into the dynamics of asthma Inhaled corticosteroid management. Further to asthma control and quality of life, self-reported side effects of treat- questionnaire; ment can be assessed with the validated Inhaled Corticosteroid Questionnaire (ICQ). ICQ; Objectives: To compare patient-reported side effects between the inhaled corticosteroids Patient-reported ciclesonide and fluticasone propionate. outcomes Methods: Patients with moderate or moderate-to-severe asthma, pre-treated with a constant dose and type of medication, were randomized in three separate studies: 1) once daily cicle- sonide 320 mg(n Z 234) or twice daily fluticasone propionate 200 mg(n Z 240); 2) twice daily ciclesonide 320 mg(n Z 255) or twice daily fluticasone propionate 375 mg(n Z 273); and 3) twice daily ciclesonide 320 mg(n Z 259) or twice daily fluticasone propionate 500 mg (n Z 244). -

Inhaled Corticosteroid (ICS) Only – Metered Dose Inhalers (MDI)
Categorisation and Formulary Choices of Inhaled Corticosteroids for Adults Inhaled Corticosteroid (ICS) only – Metered Dose Inhalers (MDI) MDI Beclometasone dipropionate Use with a compatible Fluticasone Propionate spacer device Prescribe by BRAND Clenil Modulite® Qvar®(extrafine) Qvar®(extrafine) Flixotide® Evohaler MDI Easi-Breathe Low Dose 100mcg, two puffs twice a 50 mcg, two puffs twice a day 50 mcg, two puffs twice a day 50 mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) ≤400mcg BDP) £4.45/month £4.72/month £4.64/month £5.44/month Medium Dose 200mcg, two puffs twice a 100 mcg, two puffs twice a day 100 mcg, two puffs twice a day 125mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) 400-1000mcg BDP) £9.70/month £10.33/month £10.17/month £12.50/month High Dose 250mcg, two puffs twice a 100 mcg, four puffs twice a day 100 mcg, four puffs twice a day 250mcg, two puffs twice a day (BDP equivalent day (200 dose) (200 dose) (200 dose) (120 dose) >1000mcg BDP) £9.77/month £20.66/month £20.34/month £20.00/month Simultaneous specialist OR referral recommended 250mcg four puffs, twice a day (200 dose) £19.55/month Prices correct as per Drug Tariff and DM&D December2018 1st line choices (dark green, solid border) 2nd line choices (light green, dashed border) Approved by Hertfordshire Medicines Management Committee – October 2018 Inhaled Corticosteroid (ICS) only – Dry Powder Inhalers (DPI) Beclometasone DPI dipropionate Budesonide Fluticasone Propionate Prescribe -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TRELEGY ELLIPTA 100/62.5/25 fluticasone furoate (100 micrograms)/umeclidinium (as bromide) (62.5 micrograms)/vilanterol (as trifenatate) (25 micrograms), powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 92 micrograms fluticasone furoate, 55 micrograms umeclidinium (equivalent to 65 micrograms umeclidinium [as bromide]) and 22 micrograms vilanterol (as trifenatate). This corresponds to a pre-dispensed dose of 100 micrograms fluticasone furoate, 62.5 micrograms umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide) and 25 micrograms vilanterol (as trifenatate). Excipient with known effect: Each delivered dose contains approximately 25 milligrams of lactose (as monohydrate). For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for inhalation. White powder in a light grey inhaler (Ellipta) with a beige mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications TRELEGY ELLIPTA is indicated for the maintenance treatment of adults with moderate to severe chronic obstructive pulmonary disease (COPD) who require treatment with a long- acting muscarinic receptor antagonist (LAMA) + long-acting beta2-receptor agonist (LABA) + inhaled corticosteroid (ICS). TRELEGY ELLIPTA should not be used for the initiation of COPD treatment. 4.2. Dose and method of administration Patients can be changed from their existing inhalers to TRELEGY ELLIPTA at the next dose. However it is important that patients do not take other LABA or LAMA or ICS while taking TRELEGY ELLIPTA. A stepwise approach to the management of COPD is recommended, including the cessation of smoking and a pulmonary rehabilitation program. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM.