Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
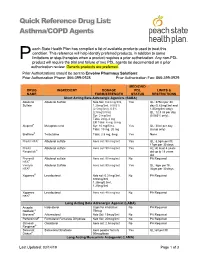
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

COPD: Treatment Updates and Transitions of Care
COPD: Treatment Updates and Transitions of Care PRESENTED AS A LIVE WEBINAR ON-DEMAND ACTIVITY Thursday, May 21, 2020 Release date: 5/31/2020 1:00 p.m. - 2:00 p.m. Expiration date: 5/31/2023 FACULTY Dennis M. Williams, Pharm.D., BCPS, AE-C, FASHP, FCCP, FAPhA Associate Professor Division of Pharmacotherapy and Experimental Therapeutics UNC Eshelman School of Pharmacy University of North Carolina Chapel Hill, North Carolina Bradley Drummond, M.D., MHS Associate Professor of Medicine University of North Carolina at Chapel Hill Chapel Hill, North Carolina View faculty bios at https://www.copdcare.org/faculty-bios.php ASHP FINANCIAL RELATIONSHIP DISCLOSURE STATEMENT Planners, presenters, reviewers, ASHP staff, and others with an opportunity to control CE content are required to disclose relevant financial relationships with ACCME-defined commercial interests. All actual conflicts of interest have been resolved prior to the continuing education activity taking place. ASHP will disclose financial relationship information prior to the beginning of the activity. A relevant financial relationship is a defined as a financial relationship between an individual (or spouse/partner) in control of content and a commercial interest, in any amount, in the past 12 months, and products and/or services of the commercial interest (with which they have the financial relationship) are related to the continuing education activity. An ACCME-defined commercial interest is any entity producing, marketing re-selling, or distributing healthcare goods or services consumed by, or used on, patients. The ACCME does not consider providers of clinical serve directly to patients to be commercial interests—unless the provider of clinical service is owned, or controlled by, an ACCME-defined commercial interest. -

3364-136-03-01 Preparation and Administration of Medications Used in Respiratory Care Page 2
Name of Policy: Preparation and Administration of Medications Used in Respiratory Care Policy Number: 3364-136-03-01 Department: Respiratory Care Approving Officer: Associate VP Patient Care Services / CNO Responsible Agent: Director, Respiratory Care Scope: Effective Date: 6/1/2020 The University of Toledo Medical Center Initial Effective Date: 5/7/1989 Respiratory Care Department New policy proposal X Minor/technical revision of existing policy Major revision of existing policy Reaffirmation of existing policy (A) Policy Statement Medications administered by persons in the Respiratory Care Department will be in accordance with the physician's order as described in Hospital Policy # 3364-100-70-10 “Medication Management”, Pharmacy policies #3364-133-28 “Use of single and multi-dose Vials”, and # 3364-133-70 “Standard Medication Administration Times”. Medications for Respiratory Care administration must be approved by the Medical Director of Respiratory Care. Approved drugs are listed in attached Appendix A for 3364-136-03-01. (B) Purpose of Policy To insure safe preparation and administration of medications used by the practitioners of the Respiratory Care Department. (C) Procedure I. Procedure for Preparing Medications for Patient Use: Unit dose: . Unit dose medications will be used when available in ordered doses. Medications removed from the AcuDose Medication System and not administered must be returned to the AcuDose using the return medication procedure. Persons administering medications for respiratory care purposes will be knowledgeable about the drug being administered, regarding its purpose, indication, contraindication, and side affects. II. Medication Errors: The on-line SafetyNet system must be used for occurrences if: . A medication is missed. -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

Or Should It Be Mast Cell Mediator Disorders?
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Expert Rev Manuscript Author Clin Immunol Manuscript Author . Author manuscript; available in PMC 2020 February 06. Published in final edited form as: Expert Rev Clin Immunol. 2019 June ; 15(6): 639–656. doi:10.1080/1744666X.2019.1596800. Recent advances in our understanding of mast cell activation – or should it be mast cell mediator disorders? Theoharis C. Theoharidesa,b,c,d, Irene Tsilionia, Huali Rene aMolecular Immunopharmacology and Drug Discovery Laboratory, Department of Immunology, Tufts University School of Medicine, Boston, MA, USA bSackler School of Graduate Biomedical Sciences, Tufts University School of Medicine, Boston, MA, USA cDepartment of Internal Medicine, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA dDepartment of Psychiatry, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA eDepartment of Otolaryngology, Beijing Electric Power Hospital, Beijing, China Abstract Introduction: An increasing number of patients present with multiple symptoms affecting many organs including the brain due to multiple mediators released by mast cells. These unique tissue immune cells are critical for allergic reactions triggered by immunoglobulin E (IgE), but are also stimulated (not activated) by immune, drug, environmental, food, infectious, and stress triggers, leading to secretion of multiple mediators often without histamine and tryptase. The presentation, diagnosis, and management of the spectrum of mast cell disorders are very confusing. As a result, specialists have recently excluded neuropsychiatric symptoms, and made the diagnostic criteria stricter, at the expense of excluding most patients. Areas covered: A literature search was performed on papers published between January 1990 and November 2018 using MEDLINE. -

Efficacy of Revefenacin, a Long-Acting Muscarinic Antagonist for Nebulized Therapy, in Patients with Markers of More Severe COPD: a Post Hoc Subgroup Analysis James F
Donohue et al. BMC Pulmonary Medicine (2020) 20:134 https://doi.org/10.1186/s12890-020-1156-4 RESEARCH ARTICLE Open Access Efficacy of revefenacin, a long-acting muscarinic antagonist for nebulized therapy, in patients with markers of more severe COPD: a post hoc subgroup analysis James F. Donohue1, Edward Kerwin2, Chris N. Barnes3, Edmund J. Moran3, Brett Haumann3 and Glenn D. Crater3* Abstract Background: Revefenacin, a once-daily, long-acting muscarinic antagonist delivered via standard jet nebulizer, increased trough forced expiratory volume in 1 s (FEV1) in patients with moderate to very severe chronic obstructive pulmonary disease (COPD) in prior phase 3 trials. We evaluated the efficacy of revefenacin in patients with markers of more severe COPD. Methods: A post hoc subgroup analysis of two replicate, randomized, phase 3 trials was conducted over 12 weeks. Endpoints included least squares change from baseline in trough FEV1, St. George’s Respiratory Questionnaire (SGRQ) responders, and transition dyspnea index (TDI) responders at Day 85. This analysis included patient subgroups at high risk for COPD exacerbations and compared patients who received revefenacin 175 μg and placebo: severe and very severe airflow limitation (percent predicted FEV1 30%–< 50% and < 30%), 2011 Global Initiative for Chronic Obstructive Lung Disease (GOLD) D, reversibility (≥ 12% and ≥ 200 mL increase in FEV1)to short-acting bronchodilators, concurrent use of long-acting β agonists and/or inhaled corticosteroids, older age (> 65 and > 75 years), and comorbidity risk factors. Results: Revefenacin demonstrated significant improvements in FEV1 versus placebo at Day 85 among the intention-to-treat (ITT) population and all subgroups. -

FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting
FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting August 31, 2020 sNDA 209482: fluticasone furoate/umeclidinium/vilanterol fixed dose combination to reduce all-cause mortality in patients with chronic obstructive pulmonary disease NDA209482/S-0008 PADAC Clinical and Statistical Briefing Document Fluticasone furoate/umeclidinium/vilanterol fixed dose combination for all-cause mortality DISCLAIMER STATEMENT The attached package contains background information prepared by the Food and Drug Administration (FDA) for the panel members of the advisory committee. The FDA background package often contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought the supplemental New Drug Application (sNDA) 209482, for fluticasone furoate/umeclidinium/vilanterol, as an inhaled fixed dose combination, for the reduction in all-cause mortality in patients with COPD, to this Advisory Committee in order to gain the Committee’s insights and opinions, and the background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered -

Utah Medicaid Pharmacy and Therapeutics Committee Drug
Utah Medicaid Pharmacy and Therapeutics Committee Drug Class Review Single Ingredient Nasal Corticosteroids Beclomethasone dipropionate (Qnasl) Beclomethasone dipropionate monohydrate (Beconase AQ) Budesonide (Rhinocort) Ciclesonide (Omnaris, Zetonna) Flunisolide (Generic) Fluticasone Furoate (Flonase Sensimist) Fluticasone Propionate (Flonase, Xhance) Mometasone Furoate (Nasonex) Triamcinolone Acetonide (Nasacort) AHFS Classification: 52.08.08 Corticosteroids (EENT) Final Report February 2018 Review prepared by: Valerie Gonzales, Pharm.D., Clinical Pharmacist Elena Martinez Alonso, B.Pharm., MSc MTSI, Medical Writer Vicki Frydrych, Pharm.D., Clinical Pharmacist Joanita Lake, B.Pharm., MSc EBHC (Oxon), Research Assistant Professor Joanne LaFleur, Pharm.D., MSPH, Associate Professor University of Utah College of Pharmacy University of Utah College of Pharmacy, Drug Regimen Review Center Copyright © 2018 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved Contents Abbreviations ................................................................................................................................................ 2 Executive Summary ....................................................................................................................................... 3 Introduction .................................................................................................................................................. 5 Table 1. Nasal corticosteroid products ............................................................................................. -

HIM.PA.153 Inhaled Agents for Asthma and COPD
Clinical Policy: Inhaled Agents for Asthma and COPD Reference Number: HIM.PA.153 Effective Date: 03.01.21 Last Review Date: 02.21 Line of Business: HIM Revision Log See Important Reminder at the end of this policy for important regulatory and legal information. Description The following are inhaled agents for asthma and/or chronic obstructive pulmonary disease (COPD) requiring prior authorization: • Short acting beta-2 agonist (SABA): albuterol (ProAir® Digihaler®), levalbuterol (Xopenex® HFA, Xopenex® inhalation solution) • Inhaled corticosteroid (ICS): budesonide (Pulmicort Respules®)*, ciclesonide (Alvesco®), fluticasone (Armonair® Digihaler™), mometasone (Asmanex® HFA, Asmanex® Twisthaler®) • Long acting beta-2 agonist (LABA): arformoterol (Brovana®), formoterol (Perforormist) • Long acting muscarinic antagonist (LAMA): aclidinium bromide (Tudorza® Pressair®), glycopyrrolate (Seebri™ Neohaler®, Lonhala® Magnair®), revefenacin (Yupelri®) • Combination ICS/LABA: budesonide/formoterol (Symbicort®)*, fluticasone/salmeterol (Advair Diskus®*, AirDuo® Digihaler™, AirDuo® RespiClick®), mometasone/formoterol (Dulera®) • Combination LABA/LAMA: aclidnium/formoterol (Duaklir® Pressair®), indacaterol/glycopyrrolate (Utibron™ Neohaler®), tiotropium/olodaterol (Stiolto® Respimat®) • Combination ICS/LAMA/LABA: budesonide/glycopyrrolate/formoterol (Breztri Aerosphere™) _______________ *Generic agents do not require prior authorization. FDA Approved Indication(s) ProAir Digihaler and Xopenex are indicated for the treatment or prevention of bronchospasm -

Drug Class Review Nasal Corticosteroids
Drug Class Review Nasal Corticosteroids Final Report Update 1 June 2008 The Agency for Healthcare Research and Quality has not yet seen or approved this report The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Dana Selover, MD Tracy Dana, MLS Colleen Smith, PharmD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Marian McDonagh, PharmD, Principal Investigator, Drug Effectiveness Review Project Copyright © 2008 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 1 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION ..........................................................................................................................5 Scope and Key Questions .......................................................................................................................7 METHODS ....................................................................................................................................9 Literature Search .....................................................................................................................................9 -

Formulary (List of Covered Drugs)
2018 Formulary (List of Covered Drugs) PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN Assurance Rx (HMO-POS) Essence Rx (HMO-POS) Esteem Rx (HMO-POS) Promise Rx (HMO-POS) Spirit Rx (HMO-POS) Surety Rx (HMO-POS) This formulary was updated on Nov. 21, 2018. For more recent information or other questions, please contact Security Health Plan Customer Service at 1-877-998-0998 or, for TTY users, 711, or visit https://www.securityhealth.org/medicareformulary. We are open 7 days a week, 8 a.m. to 8 p.m., from Oct. 1-March 31; and Monday through Friday, 8 a.m. to 8 p.m., from April 1-Sept. 30. Y0117_MC-778-0628-C-09-18 Effective date 12/1/2018 Updated 11/21/2018 Formulary ID 00018450, v.20 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us,” or “our,” it means Security Health Plan. When it refers to “plan” or “our plan,” it means Assurance Rx (HMO-POS), Essence Rx (HMO-POS), Promise Rx (HMO-POS), Spirit Rx (HMO- POS) or Surety Rx (HMO-POS). This document includes a list of the drugs (formulary) for our plan which is current as of Dec. 1, 2018. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. -

Chapter 10 Drugs for Treating Colds and Allergies Case Study It Seems
Chapter 10 Drugs for Treating Colds and Allergies Case Study It seems that the university’s wrestling team benefited from the exceptionally long break the coach gave them over the Christmas holidays because they all seem eager to continue the remaining part of the season in earnest. However, Sam, the team’s 74-kg class wrestler, appears uncomfortable and less energetic. He reports that over break he contracted an upper respiratory tract infection. Sam started taking an over-the-counter (OTC) medication to relieve his runny nose when he first felt the onset of the cold. He states that it seems to be helping, but he is feeling more tired since starting the medication. What is a possible explanation for Sam’s symptoms? Answer: The medication Sam is taking likely contains a first-generation antihistamine. These medications are found in several OTC cough, cold, and allergy products, both alone and in combination with other medications. They are helpful in colds because they decrease mucus production and have a drying effect, therefore relieving the symptom of runny nose. However, they also have a major adverse effect of causing drowsiness. This is likely the cause of Sam’s tiredness. The second-generation antihistamines are non-sedating; however, they also lack the drying effect of the first-generation antihistamines and therefore would not be as effective in treating Sam’s symptoms. Some of the first-generation antihistamines, such as chlorpheniramine, have less of a sedating effect, so switching products may help with the adverse effects. Exam Questions 1. All of the following intranasal corticosteroid products are available by prescription only except for: a.