1 Class Review: Intranasal Allergy Drugs Month/Year of Review
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

3364-136-03-01 Preparation and Administration of Medications Used in Respiratory Care Page 2
Name of Policy: Preparation and Administration of Medications Used in Respiratory Care Policy Number: 3364-136-03-01 Department: Respiratory Care Approving Officer: Associate VP Patient Care Services / CNO Responsible Agent: Director, Respiratory Care Scope: Effective Date: 6/1/2020 The University of Toledo Medical Center Initial Effective Date: 5/7/1989 Respiratory Care Department New policy proposal X Minor/technical revision of existing policy Major revision of existing policy Reaffirmation of existing policy (A) Policy Statement Medications administered by persons in the Respiratory Care Department will be in accordance with the physician's order as described in Hospital Policy # 3364-100-70-10 “Medication Management”, Pharmacy policies #3364-133-28 “Use of single and multi-dose Vials”, and # 3364-133-70 “Standard Medication Administration Times”. Medications for Respiratory Care administration must be approved by the Medical Director of Respiratory Care. Approved drugs are listed in attached Appendix A for 3364-136-03-01. (B) Purpose of Policy To insure safe preparation and administration of medications used by the practitioners of the Respiratory Care Department. (C) Procedure I. Procedure for Preparing Medications for Patient Use: Unit dose: . Unit dose medications will be used when available in ordered doses. Medications removed from the AcuDose Medication System and not administered must be returned to the AcuDose using the return medication procedure. Persons administering medications for respiratory care purposes will be knowledgeable about the drug being administered, regarding its purpose, indication, contraindication, and side affects. II. Medication Errors: The on-line SafetyNet system must be used for occurrences if: . A medication is missed. -

Or Should It Be Mast Cell Mediator Disorders?
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Expert Rev Manuscript Author Clin Immunol Manuscript Author . Author manuscript; available in PMC 2020 February 06. Published in final edited form as: Expert Rev Clin Immunol. 2019 June ; 15(6): 639–656. doi:10.1080/1744666X.2019.1596800. Recent advances in our understanding of mast cell activation – or should it be mast cell mediator disorders? Theoharis C. Theoharidesa,b,c,d, Irene Tsilionia, Huali Rene aMolecular Immunopharmacology and Drug Discovery Laboratory, Department of Immunology, Tufts University School of Medicine, Boston, MA, USA bSackler School of Graduate Biomedical Sciences, Tufts University School of Medicine, Boston, MA, USA cDepartment of Internal Medicine, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA dDepartment of Psychiatry, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA eDepartment of Otolaryngology, Beijing Electric Power Hospital, Beijing, China Abstract Introduction: An increasing number of patients present with multiple symptoms affecting many organs including the brain due to multiple mediators released by mast cells. These unique tissue immune cells are critical for allergic reactions triggered by immunoglobulin E (IgE), but are also stimulated (not activated) by immune, drug, environmental, food, infectious, and stress triggers, leading to secretion of multiple mediators often without histamine and tryptase. The presentation, diagnosis, and management of the spectrum of mast cell disorders are very confusing. As a result, specialists have recently excluded neuropsychiatric symptoms, and made the diagnostic criteria stricter, at the expense of excluding most patients. Areas covered: A literature search was performed on papers published between January 1990 and November 2018 using MEDLINE. -

Chapter 10 Drugs for Treating Colds and Allergies Case Study It Seems
Chapter 10 Drugs for Treating Colds and Allergies Case Study It seems that the university’s wrestling team benefited from the exceptionally long break the coach gave them over the Christmas holidays because they all seem eager to continue the remaining part of the season in earnest. However, Sam, the team’s 74-kg class wrestler, appears uncomfortable and less energetic. He reports that over break he contracted an upper respiratory tract infection. Sam started taking an over-the-counter (OTC) medication to relieve his runny nose when he first felt the onset of the cold. He states that it seems to be helping, but he is feeling more tired since starting the medication. What is a possible explanation for Sam’s symptoms? Answer: The medication Sam is taking likely contains a first-generation antihistamine. These medications are found in several OTC cough, cold, and allergy products, both alone and in combination with other medications. They are helpful in colds because they decrease mucus production and have a drying effect, therefore relieving the symptom of runny nose. However, they also have a major adverse effect of causing drowsiness. This is likely the cause of Sam’s tiredness. The second-generation antihistamines are non-sedating; however, they also lack the drying effect of the first-generation antihistamines and therefore would not be as effective in treating Sam’s symptoms. Some of the first-generation antihistamines, such as chlorpheniramine, have less of a sedating effect, so switching products may help with the adverse effects. Exam Questions 1. All of the following intranasal corticosteroid products are available by prescription only except for: a. -

Comparison of Long-Term Clinical Implications of Beta-Blockade In
Comparison of long-term clinical implications of beta-blockade in patients with obstructive airway diseases exposed to beta-blockers with different 1-adrenoreceptor selectivity An Italian population-based cohort study Sessa, Maurizio; Mascolo, Annamaria; Scavone, Cristina; Perone, Ilaria; Giorgio, Annalisa Di; Tari, Michele; Fucile, Annamaria; De Angelis, Antonella; Rasmussen, Daniel Bech; Jensen, Magnus Thorsten; Kragholm, Kristian; Rossi, Francesco; Capuano, Annalisa; Sportiello, Liberata Published in: Frontiers in Pharmacology DOI: 10.3389/fphar.2018.01212 Publication date: 2018 Document version Publisher's PDF, also known as Version of record Citation for published version (APA): Sessa, M., Mascolo, A., Scavone, C., Perone, I., Giorgio, A. D., Tari, M., Fucile, A., De Angelis, A., Rasmussen, D. B., Jensen, M. T., Kragholm, K., Rossi, F., Capuano, A., & Sportiello, L. (2018). Comparison of long-term clinical implications of beta-blockade in patients with obstructive airway diseases exposed to beta-blockers with different 1-adrenoreceptor selectivity: An Italian population-based cohort study. Frontiers in Pharmacology, 9, 1- 8. [1212]. https://doi.org/10.3389/fphar.2018.01212 Download date: 26. Sep. 2021 fphar-09-01212 October 23, 2018 Time: 14:25 # 1 ORIGINAL RESEARCH published: 25 October 2018 doi: 10.3389/fphar.2018.01212 Comparison of Long-Term Clinical Implications of Beta-Blockade in Patients With Obstructive Airway Diseases Exposed to Beta-Blockers With Different b1-Adrenoreceptor Edited by: Lei Xi, Selectivity: An Italian -

Combination Long-Acting Beta-Agonist Inhaled Corticosteroid: Summary of Clinical Evidence and Drug Utilization Evaluation
Drug Use Research & Management Program DHS Division of Medical Assistance Programs, 500 Summer Street NE, E35; Salem, OR 97301-1079 Phone 503-947-5220 | Fax 503-947-1119 Combination Long-Acting Beta-Agonist Inhaled Corticosteroid: Summary of Clinical Evidence and Drug Utilization Evaluation Currently, there are two FDA approved combination long-acting beta agonist (LABA) inhaled corticosteroid (ICS) products on the market: salmeterol / fluticasone propionate (Advair diskus, Advair HFA), and formoterol fumarate dehydrate / budesonide (Symbicort). Both drugs are approved for the treatment of asthma and chronic obstructive pulmonary disease (COPD). Specifically, Advair is indicated for treatment of asthma in patients 4 years and older and treatment of airflow obstruction and reducing exacerbations in patients with COPD. Symbicort is approved for maintenance treatment of asthma in patients 12 years of age and older and treatment of airflow obstruction in patients with COPD. Both agents carry an FDA black box warning related to the excess risk of asthma-related death conferred by the LABA component. The FDA issued a Public Health Advisory and revised the product labeling in November 2005 and March 2006 respectively. In February 2010, the FDA recommended additional labeling changes based on a joint meeting of the Pulmonary Allergy Drugs, Drug Safety and Risk Management, and Pediatric Advisory Committees in December 2008. Summaries of combined LABA-ICS evidence of effectiveness and safety are provided below. Evidence for these summaries was -
Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS)
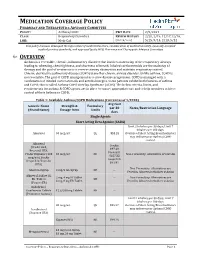
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 2/9/2021 CLASS: Respiratory Disorders REVIEW HISTORY 2/20, 2/19, 12/17,12/16, LOB: Medi-Cal (MONTH/YEAR) 5/15, 9/14, 2/13, 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 1/2020) Avg Cost Generic Name Strength & Formulary per 30 Notes/Restriction Language (Brand Name) Dosage form Limits days Single Agents Short Acting Beta Agonist (SABA) Limit 2 inhalers per 30 days; Limit 7 inhalers per 180 days. Albuterol 90 mcg/act QL $53.28 Overuse of Short Acting Bronchodilators may indicate poor Asthma/COPD control. -

Effect of Beclomethasone Dipropionate Versus Mast Cell Stabilizer on Mucociliary Clearance in Patients of Allergic Rhinitis
Open Access ORIGINAL ARTICLE Full Length A rt icl e Effect of Beclomethasone Dipropionate Versus Mast Cell Stabilizer on Mucociliary Clearance in Patients of Allergic Rhinitis Muhammad Ramzan 1, Nasir Iqbal 2, Arslan Karim 3 1 Ex House officer Nishtar Hospital Multan 2 Ex House officer, Medical officer BHU Wandher Multan 3 Ex House officer, Medical officer BHU Qadir Pur Multan ABSTRACT Objective: The study was conducted to compare the effect of Beclomethasone dipropionate with mast cell stabilizer on mucociliary clearance in patients of allergic rhinitis. Patients and Methods: A randomized trial was carried out in ENT department of Nishtar Hospital Multan from December 2016 to February 2017. Patients of allergic rhinitis from both genders were included in the study after written consent. Mucociliary transport velocity was measured by saccharine dye test. The collected data was processed and analyzed by SPSS software 23 version and presented in tabulation form. Qualitative variables were analyzed in percentage and frequency form. The main outcome variable, mucociliary transport velocity, age and weight of patients were presented as mean and stranded deviation. Students’ test was applied to calculate p value. P value <0.05 was taken as significant. Results: A total number of 140 patients were included in the study and were divided in two main groups. There were 70 volunteers in each group. One group was provided Beclomethasone and other group was given mast cell stabilizer for one week. After careful analysis and calculation, it was observed that the mean of mucociliary transport velocity after one week of Beclomethasone dipropionate treatment was 7.33±1.27 mm/minute with p value 0.558 and mean of velocity after mast cell stabilizer (Nedocromil sodium) was 7.82±1.2 mm/minute with p-values 0.304. -

An Essential Role for Mast Cells As Modulators of Neutrophils Influx In
Laboratory Investigation (2011) 91, 33–42 & 2011 USCAP, Inc All rights reserved 0023-6837/11 $32.00 An essential role for mast cells as modulators of neutrophils influx in collagen-induced arthritis in the mouse Tatiana Aparecida Pimentel1,5, Andre´ Luiz Franco Sampaio2,3,5, Fulvio D’Acquisto2, Mauro Perretti2 and Sonia Maria Oliani1,4 Mast cells are involved in immune disorders so that many of the proinflammatory and tissue destructive mediators produced by these cells have been implicated in the pathogenesis of rheumatoid arthritis. This scenario prompted us to investigate the correlation between mast cell degranulation and neutrophil influx within the digits and knees joints of arthritic mice assessing what could be the functional role(s) of joint mast cells in the response to collagen immunization. DBA/1J mice were submitted to collagen-induced arthritis and disease was assessed on day 21, 32 and 42 post-immunization. Pharmacological treatment with the glucocorticoid prednisolone, commonly used in the clinic, and nedocromil, a mast cell stabilizer, was performed from day 21 to 30. Arthritis develop after immunization, gradually increased up to day 42. Neutrophil infiltration peaked on day 32 and 21, in the digits and knees, respectively, showing an unequal pattern of recruitment between these tissues. This difference emerged for mast cells: they peaked in the digits on day 21, but a higher degree of degranulation could be measured in the knee joints. Uneven modulation of arthritis occurred after treatment of mice with prednisolone or nedocromil. Neutrophils migration to the tissue was reduced after both therapies, but only prednisolone augmented mast cell migration to the joints. -

The Mast Cell Disease Primer
The Mastocytosis Society, Inc. (TMS) (Changing to The Mast Cell Disease Society, Inc. effective June 30, 2020) Presents The Mast Cell Disease Primer Valerie M. Slee, RN, BSN, Chair Susan Jennings, PhD, Research Chair Jan Hempstead, RN, Vice-Chair, Patient Care Coordination Chair www.tmsforacure.org © 2020 The Mastocytosis Society, Inc. What are Mast Cells? • Mast cells are immune system cells that live in the bone marrow and in body tissues, internal and external, such as the gastrointestinal tract, the lining of the airway and the skin. • Mast cells are involved in allergic reactions. • Mast cells have within them small “sacs” surrounded by membranes. Mast cell granule (sac) which contains mediators Mast cell (electron micrograph) www.tmsforacure.org © 2020 The Mastocytosis Society, Inc. What are Mediators? • The sacs within mast cells (granules) contain many different kinds of substances called mediators, which participate in allergic or other reactions and anaphylaxis. • Those mediators are normally selectively released when there is an allergic or mast cell-based reaction. Gilfillan AM, et al. Adv Exp Med Biol. 2011;716:2-12. www.tmsforacure.org © 2020 The Mastocytosis Society, Inc. Possible Effects of Some Mast Cell Mediators MEDIATORS POSSIBLE EFFECTS Histamine Flushing, itching, diarrhea, hypotension Leukotrienes Shortness of breath Prostaglandins Flushing, bone pain, brain fog, cramping Tryptase Osteoporosis, skin lesions Interleukins Fatigue, weight loss, enlarged lymph nodes Heparin Osteoporosis, problems with clotting/bleeding Tumor Necrosis Factor-α Fatigue, headaches, body aches Carter MC, et al. Immunol Allergy Clin North Am. 2014;34(1):181-96. Theoharides TC, et al. N Engl J Med. 2015;373(2):163-72. -

WO 2015/120389 Al 13 August 2015 (13.08.2015) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2015/120389 Al 13 August 2015 (13.08.2015) P O P C T (51) International Patent Classification: AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, A61K 31/35 (2006.01) A61M 16/10 (2006.01) BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, A61P 37/08 (2006.01) DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (21) International Application Number: KZ, LA, LC, LK, LR, LS, LU, LY, MA, MD, ME, MG, PCT/US20 15/0 15029 MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, OM, (22) International Filing Date: PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, SC, ' February 2015 (09.02.2015) SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, TN, TR, TT, TZ, UA, UG, US, UZ, VC, VN, ZA, ZM, ZW. (25) Filing Language: English (84) Designated States (unless otherwise indicated, for every (26) Publication Language: English kind of regional protection available): ARIPO (BW, GH, (30) Priority Data: GM, KE, LR, LS, MW, MZ, NA, RW, SD, SL, ST, SZ, 61/937,928 10 February 2014 (10.02.2014) US TZ, UG, ZM, ZW), Eurasian (AM, AZ, BY, KG, KZ, RU, 61/971,709 28 March 2014 (28.03.2014) US TJ, TM), European (AL, AT, BE, BG, CH, CY, CZ, DE, 61/978,71 1 11 April 2014 ( 11.04.2014) us DK, EE, ES, FI, FR, GB, GR, HR, HU, IE, IS, IT, LT, LU, LV, MC, MK, MT, NL, NO, PL, PT, RO, RS, SE, SI, SK, 62/105,423 20 January 20 15 (20.01.2015) us SM, TR), OAPI (BF, BJ, CF, CG, CI, CM, GA, GN, GQ, (71) Applicant: PATARA PHARMA, LLC [US/US]; 11455 GW, KM, ML, MR, NE, SN, TD, TG). -

Anticholinergic-Combination-Mast-Cell-Stabilizer-Oral-Inhalation-Quantity-Limit.Pdf
QUANTITY LIMIT CRITERIA DRUG CLASS ANTICHOLINERGIC, COMBINATION, AND MAST CELL STABILIZER ORAL INHALATION BRAND NAME (generic) ATROVENT HFA (ipratropium) COMBIVENT RESPIMAT (ipratropium/albuterol) (cromolyn inhalation solution) INCRUSE ELLIPTA (umeclidinium) (ipratropium inhalation solution) (ipratropium/albuterol inhalation solution) LONHALA MAGNAIR (glycopyrrolate inhalation solution) SEEBRI NEOHALER (glycopyrrolate) SPIRIVA HANDIHALER (tiotropium) SPIRIVA RESPIMAT (tiotropium) TUDORZA PRESSAIR (aclidinium) Status: CVS Caremark Criteria Type: Quantity Limit Anticholinergic,Comb,MastCell QLimit CVS Caremark is an independent company that provides pharmacy benefit management services to CareFirst BlueCross BlueShield and CareFirst BlueChoice, Inc. members. CareFirst BlueCross BlueShield is the shared business name of CareFirst of Maryland, Inc. and Group Hospitalization and Medical Services, Inc. CareFirst of Maryland, Inc., Group Hospitalization and Medical Services, Inc., CareFirst BlueChoice, Inc., The Dental Network and First Care, Inc. are independent licensees of the Blue Cross and Blue Shield Association. In the District of Columbia and Maryland, CareFirst MedPlus is the business name of First Care, Inc. In Virginia, CareFirst MedPlus is the business name of First Care, Inc. of Maryland (used in VA by: First Care, Inc.). ® Registered trademark of the Blue Cross and Blue Shield Association Page 1 of 5 POLICY FDA-APPROVED INDICATIONS Atrovent HFA Atrovent HFA Inhalation Aerosol is indicated as a bronchodilator for maintenance treatment of bronchospasm associated with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and emphysema. Combivent Respimat Combivent Respimat is indicated for use in patients with chronic obstructive pulmonary disease (COPD) on a regular aerosol bronchodilator who continue to have evidence of bronchospasm and who require a second bronchodilator. Cromolyn Inhalation Solution Cromolyn sodium inhalation solution, USP is a prophylactic agent indicated in the management of patients with bronchial asthma. -

Cytokine Storm Syndrome in SARS-Cov-2 Infections: a Functional Role of Mast Cells
cells Review Cytokine Storm Syndrome in SARS-CoV-2 Infections: A Functional Role of Mast Cells Bahareh Hafezi 1,† , Lily Chan 2,†, Jason P. Knapp 2,†, Negar Karimi 1,† , Kimia Alizadeh 3,†, Yeganeh Mehrani 2,† , Byram W. Bridle 2,*,‡ and Khalil Karimi 2,*,‡ 1 Department of Clinical Science, School of Veterinary Medicine, Ferdowsi University of Mashhad, Azadi Square, Mashhad 9177948974, Iran; [email protected] (B.H.); [email protected] (N.K.) 2 Department of Pathobiology, Ontario Veterinary College, University of Guelph, Guelph, ON N1G 2W1, Canada; [email protected] (L.C.); [email protected] (J.P.K.); [email protected] (Y.M.) 3 Department of Diagnostic Medicine & Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS 66506, USA; [email protected] * Correspondence: [email protected] (B.W.B.); [email protected] (K.K.); Tel.: +1-519-824-4120 (B.W.B. & K.K.) † These first authors contributed equally to this work. ‡ These senior authors contributed equally to this work. Abstract: Cytokine storm syndrome is a cascade of escalated immune responses disposing the immune system to exhaustion, which might ultimately result in organ failure and fatal respiratory distress. Infection with severe acute respiratory syndrome-coronavirus-2 can result in uncontrolled production of cytokines and eventually the development of cytokine storm syndrome. Mast cells may react to viruses in collaboration with other cells and lung autopsy findings from patients that died from the coronavirus disease that emerged in 2019 (COVID-19) showed accumulation of mast cells in Citation: Hafezi, B.; Chan, L.; Knapp, the lungs that was thought to be the cause of pulmonary edema, inflammation, and thrombosis.