Pharmacological Treatment Options and Tier Coverage for Copd/Asthma
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Showa Univ J Med Sci 21(2), 77~84, June 2009 Original Inhibitory Activity of Pranlukast and Montelukas Against Histamine Release and LTC4 Production from Human Basophils 1, 2 1 1 Satoshi HIBINO ), Ryoko ITO ), Taeru KITABAYASHI ), 1 2 Kazuo ITAHASHI ) and Toshio NAKADATE ) Abstract : Leukotriene receptor antagonists(LTRAs)are routinely used to treat bronchial asthma and are thought to act mostly by inhibiting leukotriene receptors. However, there is no preclinical or clinical evidence of the direct effect of LTRAs on histamine release from and leukotriene(LT)C4 produc- tion by basophils. We used anti-IgE antibody(Ab), FMLP, and C5a to induce histamine release, and anti-IgE Ab and FMLP to stimulate LTC 4 production. Basophils were exposed to different concentrations of pranlukast and montelu- kast, and then to anti-IgE Ab, FMLP, and C5a. Culture supernatant histamine and LTC 4 levels were measured by using a histamine ELISA kit and a LTC 4 EIA kit, respectively. Histamine release was expressed as a percentage of the total histamine content(%HR)induced by anti-IgE Ab, FMLP, or C5a. To evaluate the effects of pranlukast and montelukast on histamine release and LTC 4 production, we calculated the percent inhibition of histamine release and LTC 4 production, expressed as percent inhibition, at different concentrations of pranlukast and montelukast. Pranlukast significantly inhibited histamine release stimulated by FMLP and C5a, but had no effect on histamine release stimulated by anti-IgE Ab. By comparison, montelukast signicantly inhibited histamine release stimulated by FMLP, C5a, and anti-IgE Ab, in a concentration-dependent manner. Both pranlukast and montelukast signicantly inhibited LTC 4 production stimulated by anti-IgE Ab and FMLP. -

Medicaid Policy Change
MEDICAID POLICY CHANGE IMMINENT PERIL JUSTIFICATION September 25, 2019 ADVAIR: POLICY CHANGE: LDH is changing the preferred drug list to switch the diskus inhaled powder from preferred to non-preferred and adding the HFA inhaler to the preferred list instead. JUSTIFICATION: This product is used to control symptoms and prevent complications caused by asthma or chronic obstructive pulmonary disease. This change is necessary to make an easier delivery device available for recipients to aid with treatment. Without preferred status, recipients would be required to obtain prior authorization which could delay necessary treatment. This change is needed by 10/1/19 due to the coming seasonal change in weather, including influenza and allergy season, that can significantly exacerbate chronic lung diseases, and so this presents an imminent peril to public health. EFFECTIVE DATE: October 1, 2019 LA Medicaid Preferred Drug List (PDL)/Non-Preferred Drug List (NPDL) Effective Date: July 15October 1, 2019 AG – Authorized Generic DR – Concurrent Prescriptions Must Be Written by Same Prescriber PU – Prior Use of Other Medication is Required AL – Age Limits DS – Maximum Days’ Supply Allowed QL – Quantity Limits BH – Behavioral Health Clinical Authorization Required for Children Younger Than 6 DT – Duration of Therapy Limit RX – Specific Prescription Requirements Years Old BY – Diagnosis Codes Bypass Some Requirements DX – Diagnosis Code Requirements TD – Therapeutic Duplication UN – Drug Use Not Warranted (Needs Appropriate CL – More Detailed Clinical Information -

COPD: Treatment Updates and Transitions of Care
COPD: Treatment Updates and Transitions of Care PRESENTED AS A LIVE WEBINAR ON-DEMAND ACTIVITY Thursday, May 21, 2020 Release date: 5/31/2020 1:00 p.m. - 2:00 p.m. Expiration date: 5/31/2023 FACULTY Dennis M. Williams, Pharm.D., BCPS, AE-C, FASHP, FCCP, FAPhA Associate Professor Division of Pharmacotherapy and Experimental Therapeutics UNC Eshelman School of Pharmacy University of North Carolina Chapel Hill, North Carolina Bradley Drummond, M.D., MHS Associate Professor of Medicine University of North Carolina at Chapel Hill Chapel Hill, North Carolina View faculty bios at https://www.copdcare.org/faculty-bios.php ASHP FINANCIAL RELATIONSHIP DISCLOSURE STATEMENT Planners, presenters, reviewers, ASHP staff, and others with an opportunity to control CE content are required to disclose relevant financial relationships with ACCME-defined commercial interests. All actual conflicts of interest have been resolved prior to the continuing education activity taking place. ASHP will disclose financial relationship information prior to the beginning of the activity. A relevant financial relationship is a defined as a financial relationship between an individual (or spouse/partner) in control of content and a commercial interest, in any amount, in the past 12 months, and products and/or services of the commercial interest (with which they have the financial relationship) are related to the continuing education activity. An ACCME-defined commercial interest is any entity producing, marketing re-selling, or distributing healthcare goods or services consumed by, or used on, patients. The ACCME does not consider providers of clinical serve directly to patients to be commercial interests—unless the provider of clinical service is owned, or controlled by, an ACCME-defined commercial interest. -

3364-136-03-01 Preparation and Administration of Medications Used in Respiratory Care Page 2
Name of Policy: Preparation and Administration of Medications Used in Respiratory Care Policy Number: 3364-136-03-01 Department: Respiratory Care Approving Officer: Associate VP Patient Care Services / CNO Responsible Agent: Director, Respiratory Care Scope: Effective Date: 6/1/2020 The University of Toledo Medical Center Initial Effective Date: 5/7/1989 Respiratory Care Department New policy proposal X Minor/technical revision of existing policy Major revision of existing policy Reaffirmation of existing policy (A) Policy Statement Medications administered by persons in the Respiratory Care Department will be in accordance with the physician's order as described in Hospital Policy # 3364-100-70-10 “Medication Management”, Pharmacy policies #3364-133-28 “Use of single and multi-dose Vials”, and # 3364-133-70 “Standard Medication Administration Times”. Medications for Respiratory Care administration must be approved by the Medical Director of Respiratory Care. Approved drugs are listed in attached Appendix A for 3364-136-03-01. (B) Purpose of Policy To insure safe preparation and administration of medications used by the practitioners of the Respiratory Care Department. (C) Procedure I. Procedure for Preparing Medications for Patient Use: Unit dose: . Unit dose medications will be used when available in ordered doses. Medications removed from the AcuDose Medication System and not administered must be returned to the AcuDose using the return medication procedure. Persons administering medications for respiratory care purposes will be knowledgeable about the drug being administered, regarding its purpose, indication, contraindication, and side affects. II. Medication Errors: The on-line SafetyNet system must be used for occurrences if: . A medication is missed. -
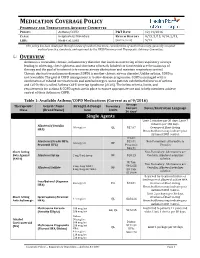
Medication Coverage Policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2016 CLASS: Respiratory Disorders REVIEW HISTORY 9/15, 5/15, 9/14, 2/13, LOB: Medi-Cal, SJHA (MONTH/YEAR) 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee. OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 9/2016) Average Therapeutic Generic Name Strength & Dosage Formulary Cost per Limits Notes/Restriction Language Class (Brand Name) form 30 days* Single Agents Limit 2 inhalers per 30 days; Limit 7 -

Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma
Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma Color Key n Four Components of Asthma Care n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 0– 4 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 5–11 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children >12 Years of Age & Adults n Long-Term Control Medications: Estimated Comparative Daily Dosages n Long-Term Control Medications: Usual Dosages n Quick-Relief Medications Guidelines are intended to be flexible. They serve as recommendations, not rigid criteria. Guidelines should be followed in most cases, but depending on the patient, and the circumstances, guidelines may need to be tailored to fit individual needs. 4750 11/18 Contents Criteria that suggest the diagnosis of asthma . 3 Goal of Therapy: Control of Asthma . 3 Four Components of Asthma Care 1. Assessment and Monitoring of Asthma Severity and Control . 4 2. Education for a Partnership in Care . 5 3. Control of Environmental Factors and Co-morbid Conditions that Affect Asthma . 5 4. Medications . 6 Bibliography . 6 Classifying Asthma Severity & Initiating Treatment in Children 0– 4 Years of Age . 7 Assessing Asthma Control & Adjusting Therapy in Children 0– 4 Years of Age . 7 Stepwise Approach for Managing Asthma in Children 0– 4 Years of Age . 8 Classifying Asthma Severity & Initiating Treatment in Children 5–11 Years of Age . 9 Assessing Asthma Control & Adjusting Therapy in Children 5–11 Years of Age . -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

COPD Agents Review – October 2020 Page 2 | Proprietary Information
COPD Agents Therapeutic Class Review (TCR) October 1, 2020 No part of this publication may be reproduced or transmitted in any form or by any means, electronic or mechanical, including photocopying, recording, digital scanning, or via any information storage or retrieval system without the express written consent of Magellan Rx Management. All requests for permission should be mailed to: Magellan Rx Management Attention: Legal Department 6950 Columbia Gateway Drive Columbia, Maryland 21046 The materials contained herein represent the opinions of the collective authors and editors and should not be construed to be the official representation of any professional organization or group, any state Pharmacy and Therapeutics committee, any state Medicaid Agency, or any other clinical committee. This material is not intended to be relied upon as medical advice for specific medical cases and nothing contained herein should be relied upon by any patient, medical professional or layperson seeking information about a specific course of treatment for a specific medical condition. All readers of this material are responsible for independently obtaining medical advice and guidance from their own physician and/or other medical professional in regard to the best course of treatment for their specific medical condition. This publication, inclusive of all forms contained herein, is intended to be educational in nature and is intended to be used for informational purposes only. Send comments and suggestions to [email protected]. October 2020 -

Montreal Protocol on Substances That Deplete the Ozone Layer
MONTREAL PROTOCOL ON SUBSTANCES THAT DEPLETE THE OZONE LAYER UNEP 2006 REPORT OF THE MEDICAL TECHNICAL OPTIONS COMMITTEE 2006 ASSESSMENT UNEP 2006 REPORT OF THE MEDICAL TECHNICAL OPTIONS COMMITTEE 2006 ASSESSMENT 2006 MTOC Assessment Report iii Montreal Protocol On Substances that Deplete the Ozone Layer Report of the UNEP Medical Technical Options Committee 2006 Assessment ASSESSMENT REPORT The text of this report is composed in Times New Roman. Co-ordination: Medical Technical Options Committee Composition and layout: Helen Tope Reproduction: UNON Nairobi Date: January 2007 Under certain conditions, printed copies of this report are available from: UNITED NATIONS ENVIRONMENT PROGRAMME Ozone Secretariat, P.O. Box 30552, Nairobi, Kenya This document is also available in portable document format from the UNEP Ozone Secretariat's website: http://ozone.unep.org/Assessment_Panels/TEAP/Reports/MTOC/ No copyright involved. This publication may be freely copied, abstracted and cited, with acknowledgement of the source of the material. ISBN: 978-92-807-2828-6 Job No: OZO/0954/NA Cover photograph © Luo Hong. iv 2006 MTOC Assessment Report Disclaimer The United Nations Environment Programme (UNEP), the Technology and Economic Assessment Panel (TEAP) Co-chairs and members, the Technical Options Committees Co-chairs and members, the TEAP Task Forces Co-chairs and members, and the companies and organisations that employ them do not endorse the performance, worker safety, or environmental acceptability of any of the technical options discussed. Every industrial operation requires consideration of worker safety and proper disposal of contaminants and waste products. Moreover, as work continues - including additional toxicity evaluation - more information on health, environmental and safety effects of alternatives and replacements will become available for use in selecting among the options discussed in this document. -

Arformoterol Tartrate) Inhalation Solution 3 15 Mcg*/2 Ml 4 *Potency Expressed As Arformoterol 5 6 for Oral Inhalation Only 7 8 WARNING: ASTHMA RELATED DEATH
® 1 BROVANA 2 (arformoterol tartrate) Inhalation Solution 3 15 mcg*/2 mL 4 *potency expressed as arformoterol 5 6 For oral inhalation only 7 8 WARNING: ASTHMA RELATED DEATH 9 Long-acting beta2-adrenergic agonists (LABA) increase the risk of asthma-related 10 death. Data from a large placebo-controlled US study that compared the safety of 11 another long-acting beta2-adrenergic agonist (salmeterol) or placebo added to usual 12 asthma therapy showed an increase in asthma-related deaths in patients receiving 13 salmeterol. This finding with salmeterol is considered a class effect of LABA, 14 including arformoterol, the active ingredient in BROVANA (see WARNINGS). The 15 safety and efficacy of BROVANA in patients with asthma have not been established. 16 All LABA, including BROVANA, are contraindicated in patients with asthma 17 without use of a long-term asthma control medication (see 18 CONTRAINDICATIONS). 19 20 DESCRIPTION 21 BROVANA (arformoterol tartrate) Inhalation Solution is a sterile, clear, colorless, 22 aqueous solution of the tartrate salt of arformoterol, the (R,R)-enantiomer of formoterol. 23 Arformoterol is a selective beta2-adrenergic bronchodilator. The chemical name for 24 arformoterol tartrate is formamide, N-[2-hydroxy-5-[(1R)-1-hydroxy-2-[[(1R)-2 25 (4-methoxyphenyl)-1-methylethyl]amino]ethyl]phenyl]-, (2R,3R)-2,3 26 dihydroxybutanedioate (1:1 salt), and its established structural formula is as follows: OH H N . HO OH CH3 HO OCH3 HOOC COOH HN H 27 O 28 The molecular weight of arformoterol tartrate is 494.5 g/mol, and its empirical formula ٠C4H6O6 (1:1 salt). -

Or Should It Be Mast Cell Mediator Disorders?
HHS Public Access Author manuscript Author ManuscriptAuthor Manuscript Author Expert Rev Manuscript Author Clin Immunol Manuscript Author . Author manuscript; available in PMC 2020 February 06. Published in final edited form as: Expert Rev Clin Immunol. 2019 June ; 15(6): 639–656. doi:10.1080/1744666X.2019.1596800. Recent advances in our understanding of mast cell activation – or should it be mast cell mediator disorders? Theoharis C. Theoharidesa,b,c,d, Irene Tsilionia, Huali Rene aMolecular Immunopharmacology and Drug Discovery Laboratory, Department of Immunology, Tufts University School of Medicine, Boston, MA, USA bSackler School of Graduate Biomedical Sciences, Tufts University School of Medicine, Boston, MA, USA cDepartment of Internal Medicine, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA dDepartment of Psychiatry, Tufts University School of Medicine and Tufts Medical Center, Boston, MA, USA eDepartment of Otolaryngology, Beijing Electric Power Hospital, Beijing, China Abstract Introduction: An increasing number of patients present with multiple symptoms affecting many organs including the brain due to multiple mediators released by mast cells. These unique tissue immune cells are critical for allergic reactions triggered by immunoglobulin E (IgE), but are also stimulated (not activated) by immune, drug, environmental, food, infectious, and stress triggers, leading to secretion of multiple mediators often without histamine and tryptase. The presentation, diagnosis, and management of the spectrum of mast cell disorders are very confusing. As a result, specialists have recently excluded neuropsychiatric symptoms, and made the diagnostic criteria stricter, at the expense of excluding most patients. Areas covered: A literature search was performed on papers published between January 1990 and November 2018 using MEDLINE. -

Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity
ID Design Press, Skopje, Republic of Macedonia Open Access Macedonian Journal of Medical Sciences. 2018 May 20; 6(5):777-781. https://doi.org/10.3889/oamjms.2018.187 eISSN: 1857-9655 Clinical Science Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity Naim Morina1, Arsim Haliti1, Ali Iljazi2, Drita Islami3, Sadi Bexheti4, Adnan Bozalija1*, Hilmi Islami3 1Department of Pharmacy, Faculty of Medicine, University of Prishtina, Prishtina, Kosovo; 2Kosovo Occupational Health Institute, Gjakovo, Kosovo; 3Department of Pharmacology, Faculty of Medicine, University of Prishtina, Kosovo; 4Department of Anatomy, Faculty of Medicine, University of Prishtina, Kosovo Abstract Citation: Morina N, Haliti A, Iljazi A, Islami D, Bexheti S, AIM: Blocking effect of leukotriene biosynthesis–zileuton and blocking the effect of phosphodiesterase enzyme– Bozalija A, Islami H. Comparison of Effect of Leukotriene diprophylline in the treatment of patients with bronchial asthma and bronchial increased reactivity, and tiotropium Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial bromide as an antagonist of the muscarinic receptor studied in this work. Hyperreactivity. Open Access Maced J Med Sci. 2018 May 20; 6(5):777-781. METHODS: Parameters of the lung function are determined with Body plethysmography. The resistance of the https://doi.org/10.3889/oamjms.2018.187 airways (Raw) was registered and measured was intrathoracic gas volume (ITGV), and specific resistance Keywords: Bronchial asthma; Zileuton; Diprophylline and tiotropium bromide (SRaw) was also calculated. For the research, administered was zileuton (tabl. 600 mg) and diprophylline (tabl. 150 mg). *Correspondence: Adnan Bozalija. Department of Pharmacy, Faculty of Medicine, University of Prishtina, Prishtina, Kosovo.