Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Information Sheet("Kusuri-No-Shiori")
Drug Information Sheet("Kusuri-no-Shiori") Internal Published: 05/2017 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment. Brand name:PRANLUKAST TABLETS 112.5mg "CEO" Active ingredient:Pranlukast hydrate Dosage form:white to pale yellow tablet, diameter: 7.5 mm, thickness: 2.7 mm Print on wrapping:(face) プランルカスト 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg (back) PRANLUKAST 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg Effects of this medicine This medicine selectively binds to leukotriene receptor and inhibits its action. It consequently suppresses increase in airway contraction, vascular permeability, mucosal edema and hypersensitivity. It is usually used to treat bronchial asthma and allergic rhinitis. However, it cannot stop the attack of bronchial asthma already in progress but prevents the asthma attack. Before using this medicine, be sure to tell your doctor and pharmacist ・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines. ・If you are pregnant or breastfeeding. ・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule (How to take this medicine) ・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional)) ・In general, for adults, take 2 tablets (225 mg of the active ingredient) at a time, twice a day after breakfast and dinner. -

Drug Class Review Controller Medications for Asthma
Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Original Report: November 2008 Daniel E. Jonas, MD, MPH Roberta C. M. Wines, MPH Marcy DelMonte, PharmD, BCPS Halle R. Amick, MSPH Tania M. Wilkins, MS Brett D. Einerson, MPH Christine L. Schuler, MD Blake A. Wynia, MPH Betsy Bryant Shilliday, Pharm.D., CDE, CPP Produced by RTI-UNC Evidence-based Practice Center Cecil G. Sheps Center for Health Services Research University of North Carolina at Chapel Hill 725 Martin Luther King Jr. Blvd, CB# 7590 Chapel Hill, NC 27599-7590 Tim Carey, M.D., M.P.H., Director Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2011 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Update 1 Report Drug Effectiveness Review Project The medical literature relating to this topic is scanned periodically. (See http://www.ohsu.edu/xd/research/centers-institutes/evidence-based-policy- center/derp/documents/methods.cfm for description of scanning process). Prior versions of this report can be accessed at the DERP website. -

Montelukast Sodium) Tablets, Chewable Tablets, and Oral
Merck Sharp & Dohme Corp., a subsidiary of o MERCK & CO., INC. Whitehouse Station, NJ 08889/ USA HIGHLIGHTS OF PRESCRIBING INFORMATION .....................DOSAGE FORMS AND STRENGTHS..................... These highlights do not include all the information needed to use . SINGULAIR 10-mg Film-Coated Tablets SINGULAIR safely and effectively. See full prescribing information . SINGULAIR 5-mg and 4-mg Chewable Tablets for SINGULAIR. SINGULAIR 4.mg Oral Granules SINGULAIRiI .............................. CONTRAINDICATIONS .............................. (montelukast sodium) tablets, chewable tablets, and oral . Hypersensitivity to any component of this product (4). granules ....................... WARNINGS AND PRECAUTIONS ....................... Initial U.S. Approval: 1998 . Do not prescribe SINGULAIR to treat an acute asthma attack. .......................... RECENT MAJOR CHANGES........................... Advise patients to have appropriate rescue medication available Warnings and Precautions, Neuropsychiatric Events (5.4) 04/2010 (5.1). Inhaled corticosteroid may be reduced gradually. Do not abruptly ...........................INDICATIONS AND USAGE ........................... substitute SINGULAIR for inhaled or oral corticosteroids (5.2). SINGULAIRiI is a leukotriene receptor antagonist indicated for: . Patients with known aspirin sensitivity should continue to avoid . Prophylaxis and chronic treatment of asthma in patients aspirin or non-steroidal anti-inflammatory agents while taking 12 months of age and older (1.1). SINGULAIR (5.3). Acute prevention of exercise-induced bronchoconstriction (EIS) in Neuropsychiatric events have been reported with SINGULAIR. patients 15 years of age and older (1.2). Instruct patients to be alert for neuropsychiatric events. Evaluate Relief of symptoms of allergic rhinitis (AR): seasonal allergic the risks and benefits of continuing treatment with SINGULAIR if rhinitis (SAR) in patients 2 years of age and older, and perennial such events occur (5.4 and 6.2). -

FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting
FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting August 31, 2020 sNDA 209482: fluticasone furoate/umeclidinium/vilanterol fixed dose combination to reduce all-cause mortality in patients with chronic obstructive pulmonary disease NDA209482/S-0008 PADAC Clinical and Statistical Briefing Document Fluticasone furoate/umeclidinium/vilanterol fixed dose combination for all-cause mortality DISCLAIMER STATEMENT The attached package contains background information prepared by the Food and Drug Administration (FDA) for the panel members of the advisory committee. The FDA background package often contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought the supplemental New Drug Application (sNDA) 209482, for fluticasone furoate/umeclidinium/vilanterol, as an inhaled fixed dose combination, for the reduction in all-cause mortality in patients with COPD, to this Advisory Committee in order to gain the Committee’s insights and opinions, and the background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered -

Understanding Interparticle Interactions in Dry Powder Inhalation: Glass Beads As an Innovative Model Carrier System
UNDERSTANDING INTERPARTICLE INTERACTIONS IN DRY POWDER INHALATION: GLASS BEADS AS AN INNOVATIVE MODEL CARRIER SYSTEM DOCTORAL THESIS SUMBITTED IN FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR IN NATURAL SCIENCES AT KIEL UNIVERSITY, GERMANY by Niklas Ludwig Renner Kiel 2017 Referee: Prof. Dr. Regina Scherließ Co-Referee: Prof. Dr. Thomas Kunze Date of Exam: 13.10.2017 Accepted for publication: 13.10.2017 Prof. Dr. Natascha Oppelt (Dean) Published research articles contributing to the present thesis: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler performance Dalby, R.N. (Ed.), RDD Europe 2017 2 (2017), 195-200 Renner, N; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Nano- and microstructured model carrier surfaces to alter dry powder inhaler performance International Journal of Pharmaceutics 518 (2017), 20-28 Conference contributions: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler per- formance Respiratory Drug Delivery, Nice, France (2017) Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. A deeper insight into the impact of chemical surface properties on inhalation perfor- mance Drug Delivery to the Lungs 27, Edinburgh, Scotland (2016) Renner, N.; Scherließ, R.; Steckel, H. Glass beads as model carriers in dry powder inhalers: the influence of chemical sur- face properties on inhalation performance International Congress on Particle Technology, Nurnberg, Germany (2016) Renner, N.; Scherließ, R.; Steckel, H. Investigating the influence of carrier surface roughness on drug delivery in DPIs 10th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Tech- nology, Glasgow, Scotland (2016) Renner, N.; Kutelova, Z.; Scherließ, R.; Steckel, H. -

The Role of 5-Lipoxygenase in Pathophysiology and Management of Neuropathic Pain
REVIEW ARTICLE THE ROLE OF 5-LIPOXYGENASE IN PATHOPHYSIOLOGY AND MANAGEMENT OF NEUROPATHIC PAIN Pascanus Lamsihar PT∗, Faldi Yaputra∗∗, Jimmy FA Barus4 and I Putu Eka Widyadharma∗∗,1 ∗Department of Neurology, Provincial Mental Hospital, West Borneo, Indonesia., ∗∗Department of Neurology, Faculty of Medicine, Udayana University-Sanglah General Hospital, Bali, Indonesia., 4Department of Neurology, Faculty of Medicine, Atma Jaya Catholic University, Jakarta-Indonesia. ABSTRACT Neuropathic pain (NP) is a pain caused by lesions in the nervous system. Several causes of NP are traumatic, metabolic disorders, ischemia, toxins, infections, immune-related, and hereditary. The pathophysiology of NP is very complicated and unknown entirely. Therefore the treatment of NP is still unsatisfactory. Recent studies believed the critical role of primary inflammatory mediators in the pathophysiology of NP especially leukotrienes (LTs). The 5- lipoxygenase enzyme (5-LOX) is an enzyme that plays a role in the metabolism of arachidonic acid into LTs. Leukotrienes (LTs) are the essential inflammatory mediators in the pathophysiology of NP. Leukotriene B4 (LTB4) can cause chemotaxis on neutrophils, lowering nociceptors threshold and may contribute to NP. Several studies believed the administration of 5-LOX inhibitors or LTs receptor antagonists could be useful in the management of NP. The purpose of this review is to summarize the involvement of 5-LOX enzyme as an essential role in the pathophysiology and management of NP. KEYWORDS 5-lipoxygenase, leukotrienes, -

The Role of Pharmacogenomics in Improving the Management of Asthma
The Role of Pharmacogenomics in Improving the Management of Asthma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kazani S, ME Wechsler, E. Israel. 2010. TThe Role of Pharmacogenomics in Improving the Management of Asthma. Journal of Allergy and Clinical Immunology 125, no. 2: 295–302. Published Version 10.1016/j.jaci.2009.12.014 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42659246 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP NIH Public Access Author Manuscript J Allergy Clin Immunol. Author manuscript; available in PMC 2011 February 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Allergy Clin Immunol. 2010 February ; 125(2): 295±302. doi:10.1016/j.jaci.2009.12.014. THE ROLE OF PHARMACOGENOMICS IN IMPROVING THE MANAGEMENT OF ASTHMA Shamsah Kazani, M.D. [Instructor in Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Michael E. Wechsler, M.D. [Assistant Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Elliot Israel, M.D. [Associate Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Abstract There is a large amount of interindividual variability in both therapeutic and adverse responses to asthma therapies. Genetic variability may account for 50–60% of this variability. -

Singulair (Montelukast Sodium)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use --------------------- DOSAGE FORMS AND STRENGTHS -------------------- SINGULAIR safely and effectively. See full prescribing information SINGULAIR 10-mg Film-Coated Tablets for SINGULAIR. SINGULAIR 5-mg and 4-mg Chewable Tablets SINGULAIR 4-mg Oral Granules (3) SINGULAIR® (montelukast sodium) Tablets, Chewable Tablets, -------------------------------CONTRAINDICATIONS ------------------------------ and Oral Granules Hypersensitivity to any component of this product (4). Initial U.S. Approval: 1998 ------------------------WARNINGS AND PRECAUTIONS----------------------- ---------------------------RECENT MAJOR CHANGES -------------------------- Do not prescribe SINGULAIR to treat an acute asthma attack Indications and Usage (5.1). Exercise-Induced Bronchoconstriction (EIB) (1.2) 03/2012 Dosage and Administration Advise patients to have appropriate rescue medication available Exercise-Induced Bronchoconstriction (EIB) (2.2) 03/2012 (5.1). Inhaled corticosteroid may be reduced gradually. Do not abruptly ----------------------------INDICATIONS AND USAGE --------------------------- substitute SINGULAIR for inhaled or oral corticosteroids (5.2). SINGULAIR is a leukotriene receptor antagonist indicated for: Patients with known aspirin sensitivity should continue to avoid Prophylaxis and chronic treatment of asthma in patients aspirin or non-steroidal anti-inflammatory agents while taking 12 months of age and older (1.1). SINGULAIR (5.3). Acute prevention of exercise-induced bronchoconstriction (EIB) in Neuropsychiatric events have been reported with SINGULAIR. patients 6 years of age and older (1.2). Instruct patients to be alert for neuropsychiatric events. Evaluate Relief of symptoms of allergic rhinitis (AR): seasonal allergic the risks and benefits of continuing treatment with SINGULAIR if rhinitis (SAR) in patients 2 years of age and older, and perennial such events occur (5.4 and 6.2). -

Montelukast and Neuropsychiatric Events in Children with Asthma: a Nested Case-Control Study
ORIGINAL ARTICLES Montelukast and Neuropsychiatric Events in Children with Asthma: A Nested Case–Control Study S. Dresden Glockler-Lauf, MPH1, Yaron Finkelstein, MD1,2,3,4,5, Jingqin Zhu, MSc1,2, Laura Y. Feldman, MPH1, and Teresa To, PhD1,2,6 Objective To examine the association between montelukast prescription and neuropsychiatric events in children with asthma. Study design A matched, nested case–control design was used to identify cases and controls from a cohort of children aged 5-18 years with physician-diagnosed asthma from 2004 to 2015, in Ontario, Canada, prescribed an asthma maintenance medication. Cases were children with a hospitalization or emergency department visit for a neuropsychiatric event. Cases were matched to up to 4 controls on birth year, year of asthma diagnosis, and sex. The exposures were dispensed prescriptions for montelukast (yes/no) and number of dispensed montelukast prescriptions in the year before the index date. Conditional logistic regression was used to measure the unadjusted OR and aOR and 95% CIs for montelukast prescription and neuropsychiatric events. Covariates in the adjusted model included sociodemographic factors and measures of asthma severity. Results In total, 898 cases with a neuropsychiatric event and 3497 matched controls were included. Children who experienced a new-onset neuropsychiatric event had nearly 2 times the odds of having been prescribed montelu- kast, compared with controls (OR 1.91, 95% CI 1.15-3.18; P = .01). Most cases presented for anxiety (48.6%) and/ or sleep disturbance (26.1%). Conclusions Children with asthma who experienced a new-onset neuropsychiatric event had nearly twice the odds of having been prescribed montelukast in the year before their event. -
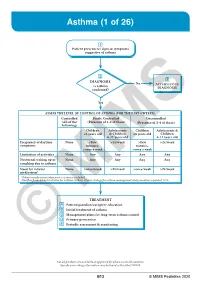
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough -

Monteleucast and Zileuton Retard the Progression of Atherosclerosis Via Down Regulation of the Inflammatory and Oxidative Pathwa
& Experim l e ca n i t in a l l C C f a Journal of Clinical & Experimental Hadi et al., J Clin Exp Cardiolog 2013, 4:6 o r d l i a o DOI: 10.4172/2155-9880.1000250 n l o r g u y o J Cardiology ISSN: 2155-9880 Research Article Open Access Monteleucast and Zileuton Retard the Progression of Atherosclerosis via Down Regulation of the Inflammatory and Oxidative Pathways Najah R Hadi1*, Bassim I Mohammad2, Ahmad Almudhafer1, Naser Yousif3 and Ahmed M Sultan2 1Kufa College of Medicine, Iraq 2Al-Qadesiyah University, Iraq 3Colorado University, USA Abstract Background: Atherosclerosis and its thrombotic complications are responsible for remarkably high numbers of deaths. Leukotrines are involved in different stages of atherosclerosis. Therefore this study was undertaken to evaluate the effect of montelukast and zileuton on the progression of atherosclerosis. Materials and methods: Thirty-five male rabbits were used in this study. These animals randomized into 5 groups (7 rabbits each). Rabbits in first group were maintained on normal rabbit chow diet and used as normal diet control group (NC). While the rabbits in other four groups were fed on atherogenic diet (2% cholesterol) for 8 weeks. The second group, Atherogenic Control Group (AC) rabbits received atherogenic diet alone. Third group, Positive Control Group (PC) rabbits received atherogenic diet and ethanol as vehicle. Forth group, Montelukast Treated Group (MT) rabbits received montelukast 1.5 mg per kg daily and the fifth group, Zileuton Treated Group (ZT) rabbits received zileuton 150 mg per kg daily. At the end of 8th weeks animals were sacrificed, blood sample was collected to measure the following parameters: lipid profile, plasma GSH, MDA, and hsCRP. -

Leukotriene Modifiers (Accolate®, Singulair®, Zileuton®)
Leukotriene Modifiers (Accolate®, Singulair®, Zileuton®) What are They? Leukotriene modifiers reduce swelling and inflammation in the airways to prevent asthma symptoms. You may not notice a change in your asthma symptoms for one to two week, after starting to use them. • These medicines will not stop a sudden asthma attack. Albuterol should be used for sudden asthma attacks. • Only regular daily use of the leukotriene modifiers will prevent asthma symptoms. • It is important that you do not stop or decrease the dose of these medications without contacting your doctor. How should they be used? • Zafirlukast (Accolate®) is a tablet that is taken by mouth twice a day on an empty stomach (1 hour before you eat or 2 hours after you eat). It is very important to take this medicine on an empty stomach. o Zafirlukast must be taken every day even if you don’t have asthma symptoms. If you forget to take your dose on time, do not take twice as much the next time. Take the regularly scheduled dose as soon as you remember, then get back to your regular schedule. o Side effects . Zafirlukast (Accolate®) causes very few side effects. Some people may have headaches, nausea, or diarrhea. Although these side effects are quite uncommon, it is important for you to let your doctor know if you are experiencing any of these while taking zafirlukast. • Montelukast (Singulair®) o A tablet that is taken once a day at nighttime. o You can take Montelukast with or without food. o Montelukast must be taken every day even if you don’t have asthma symptoms.