Antileukotriene Agents in Asthma: the Dart That Kills the Elephant?
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Drug Information Sheet("Kusuri-No-Shiori")
Drug Information Sheet("Kusuri-no-Shiori") Internal Published: 05/2017 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment. Brand name:PRANLUKAST TABLETS 112.5mg "CEO" Active ingredient:Pranlukast hydrate Dosage form:white to pale yellow tablet, diameter: 7.5 mm, thickness: 2.7 mm Print on wrapping:(face) プランルカスト 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg (back) PRANLUKAST 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg Effects of this medicine This medicine selectively binds to leukotriene receptor and inhibits its action. It consequently suppresses increase in airway contraction, vascular permeability, mucosal edema and hypersensitivity. It is usually used to treat bronchial asthma and allergic rhinitis. However, it cannot stop the attack of bronchial asthma already in progress but prevents the asthma attack. Before using this medicine, be sure to tell your doctor and pharmacist ・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines. ・If you are pregnant or breastfeeding. ・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule (How to take this medicine) ・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional)) ・In general, for adults, take 2 tablets (225 mg of the active ingredient) at a time, twice a day after breakfast and dinner. -

Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Showa Univ J Med Sci 21(2), 77~84, June 2009 Original Inhibitory Activity of Pranlukast and Montelukas Against Histamine Release and LTC4 Production from Human Basophils 1, 2 1 1 Satoshi HIBINO ), Ryoko ITO ), Taeru KITABAYASHI ), 1 2 Kazuo ITAHASHI ) and Toshio NAKADATE ) Abstract : Leukotriene receptor antagonists(LTRAs)are routinely used to treat bronchial asthma and are thought to act mostly by inhibiting leukotriene receptors. However, there is no preclinical or clinical evidence of the direct effect of LTRAs on histamine release from and leukotriene(LT)C4 produc- tion by basophils. We used anti-IgE antibody(Ab), FMLP, and C5a to induce histamine release, and anti-IgE Ab and FMLP to stimulate LTC 4 production. Basophils were exposed to different concentrations of pranlukast and montelu- kast, and then to anti-IgE Ab, FMLP, and C5a. Culture supernatant histamine and LTC 4 levels were measured by using a histamine ELISA kit and a LTC 4 EIA kit, respectively. Histamine release was expressed as a percentage of the total histamine content(%HR)induced by anti-IgE Ab, FMLP, or C5a. To evaluate the effects of pranlukast and montelukast on histamine release and LTC 4 production, we calculated the percent inhibition of histamine release and LTC 4 production, expressed as percent inhibition, at different concentrations of pranlukast and montelukast. Pranlukast significantly inhibited histamine release stimulated by FMLP and C5a, but had no effect on histamine release stimulated by anti-IgE Ab. By comparison, montelukast signicantly inhibited histamine release stimulated by FMLP, C5a, and anti-IgE Ab, in a concentration-dependent manner. Both pranlukast and montelukast signicantly inhibited LTC 4 production stimulated by anti-IgE Ab and FMLP. -
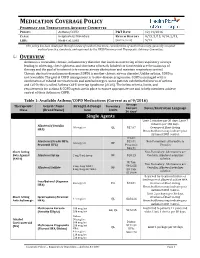
Medication Coverage Policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2016 CLASS: Respiratory Disorders REVIEW HISTORY 9/15, 5/15, 9/14, 2/13, LOB: Medi-Cal, SJHA (MONTH/YEAR) 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee. OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 9/2016) Average Therapeutic Generic Name Strength & Dosage Formulary Cost per Limits Notes/Restriction Language Class (Brand Name) form 30 days* Single Agents Limit 2 inhalers per 30 days; Limit 7 -

Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma
Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma Color Key n Four Components of Asthma Care n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 0– 4 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 5–11 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children >12 Years of Age & Adults n Long-Term Control Medications: Estimated Comparative Daily Dosages n Long-Term Control Medications: Usual Dosages n Quick-Relief Medications Guidelines are intended to be flexible. They serve as recommendations, not rigid criteria. Guidelines should be followed in most cases, but depending on the patient, and the circumstances, guidelines may need to be tailored to fit individual needs. 4750 11/18 Contents Criteria that suggest the diagnosis of asthma . 3 Goal of Therapy: Control of Asthma . 3 Four Components of Asthma Care 1. Assessment and Monitoring of Asthma Severity and Control . 4 2. Education for a Partnership in Care . 5 3. Control of Environmental Factors and Co-morbid Conditions that Affect Asthma . 5 4. Medications . 6 Bibliography . 6 Classifying Asthma Severity & Initiating Treatment in Children 0– 4 Years of Age . 7 Assessing Asthma Control & Adjusting Therapy in Children 0– 4 Years of Age . 7 Stepwise Approach for Managing Asthma in Children 0– 4 Years of Age . 8 Classifying Asthma Severity & Initiating Treatment in Children 5–11 Years of Age . 9 Assessing Asthma Control & Adjusting Therapy in Children 5–11 Years of Age . -

Drug Class Review Controller Medications for Asthma
Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Original Report: November 2008 Daniel E. Jonas, MD, MPH Roberta C. M. Wines, MPH Marcy DelMonte, PharmD, BCPS Halle R. Amick, MSPH Tania M. Wilkins, MS Brett D. Einerson, MPH Christine L. Schuler, MD Blake A. Wynia, MPH Betsy Bryant Shilliday, Pharm.D., CDE, CPP Produced by RTI-UNC Evidence-based Practice Center Cecil G. Sheps Center for Health Services Research University of North Carolina at Chapel Hill 725 Martin Luther King Jr. Blvd, CB# 7590 Chapel Hill, NC 27599-7590 Tim Carey, M.D., M.P.H., Director Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2011 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Update 1 Report Drug Effectiveness Review Project The medical literature relating to this topic is scanned periodically. (See http://www.ohsu.edu/xd/research/centers-institutes/evidence-based-policy- center/derp/documents/methods.cfm for description of scanning process). Prior versions of this report can be accessed at the DERP website. -

Montreal Protocol on Substances That Deplete the Ozone Layer
MONTREAL PROTOCOL ON SUBSTANCES THAT DEPLETE THE OZONE LAYER UNEP 2006 REPORT OF THE MEDICAL TECHNICAL OPTIONS COMMITTEE 2006 ASSESSMENT UNEP 2006 REPORT OF THE MEDICAL TECHNICAL OPTIONS COMMITTEE 2006 ASSESSMENT 2006 MTOC Assessment Report iii Montreal Protocol On Substances that Deplete the Ozone Layer Report of the UNEP Medical Technical Options Committee 2006 Assessment ASSESSMENT REPORT The text of this report is composed in Times New Roman. Co-ordination: Medical Technical Options Committee Composition and layout: Helen Tope Reproduction: UNON Nairobi Date: January 2007 Under certain conditions, printed copies of this report are available from: UNITED NATIONS ENVIRONMENT PROGRAMME Ozone Secretariat, P.O. Box 30552, Nairobi, Kenya This document is also available in portable document format from the UNEP Ozone Secretariat's website: http://ozone.unep.org/Assessment_Panels/TEAP/Reports/MTOC/ No copyright involved. This publication may be freely copied, abstracted and cited, with acknowledgement of the source of the material. ISBN: 978-92-807-2828-6 Job No: OZO/0954/NA Cover photograph © Luo Hong. iv 2006 MTOC Assessment Report Disclaimer The United Nations Environment Programme (UNEP), the Technology and Economic Assessment Panel (TEAP) Co-chairs and members, the Technical Options Committees Co-chairs and members, the TEAP Task Forces Co-chairs and members, and the companies and organisations that employ them do not endorse the performance, worker safety, or environmental acceptability of any of the technical options discussed. Every industrial operation requires consideration of worker safety and proper disposal of contaminants and waste products. Moreover, as work continues - including additional toxicity evaluation - more information on health, environmental and safety effects of alternatives and replacements will become available for use in selecting among the options discussed in this document. -

Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity
ID Design Press, Skopje, Republic of Macedonia Open Access Macedonian Journal of Medical Sciences. 2018 May 20; 6(5):777-781. https://doi.org/10.3889/oamjms.2018.187 eISSN: 1857-9655 Clinical Science Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity Naim Morina1, Arsim Haliti1, Ali Iljazi2, Drita Islami3, Sadi Bexheti4, Adnan Bozalija1*, Hilmi Islami3 1Department of Pharmacy, Faculty of Medicine, University of Prishtina, Prishtina, Kosovo; 2Kosovo Occupational Health Institute, Gjakovo, Kosovo; 3Department of Pharmacology, Faculty of Medicine, University of Prishtina, Kosovo; 4Department of Anatomy, Faculty of Medicine, University of Prishtina, Kosovo Abstract Citation: Morina N, Haliti A, Iljazi A, Islami D, Bexheti S, AIM: Blocking effect of leukotriene biosynthesis–zileuton and blocking the effect of phosphodiesterase enzyme– Bozalija A, Islami H. Comparison of Effect of Leukotriene diprophylline in the treatment of patients with bronchial asthma and bronchial increased reactivity, and tiotropium Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial bromide as an antagonist of the muscarinic receptor studied in this work. Hyperreactivity. Open Access Maced J Med Sci. 2018 May 20; 6(5):777-781. METHODS: Parameters of the lung function are determined with Body plethysmography. The resistance of the https://doi.org/10.3889/oamjms.2018.187 airways (Raw) was registered and measured was intrathoracic gas volume (ITGV), and specific resistance Keywords: Bronchial asthma; Zileuton; Diprophylline and tiotropium bromide (SRaw) was also calculated. For the research, administered was zileuton (tabl. 600 mg) and diprophylline (tabl. 150 mg). *Correspondence: Adnan Bozalija. Department of Pharmacy, Faculty of Medicine, University of Prishtina, Prishtina, Kosovo. -

FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting
FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting August 31, 2020 sNDA 209482: fluticasone furoate/umeclidinium/vilanterol fixed dose combination to reduce all-cause mortality in patients with chronic obstructive pulmonary disease NDA209482/S-0008 PADAC Clinical and Statistical Briefing Document Fluticasone furoate/umeclidinium/vilanterol fixed dose combination for all-cause mortality DISCLAIMER STATEMENT The attached package contains background information prepared by the Food and Drug Administration (FDA) for the panel members of the advisory committee. The FDA background package often contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought the supplemental New Drug Application (sNDA) 209482, for fluticasone furoate/umeclidinium/vilanterol, as an inhaled fixed dose combination, for the reduction in all-cause mortality in patients with COPD, to this Advisory Committee in order to gain the Committee’s insights and opinions, and the background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered -

Understanding Interparticle Interactions in Dry Powder Inhalation: Glass Beads As an Innovative Model Carrier System
UNDERSTANDING INTERPARTICLE INTERACTIONS IN DRY POWDER INHALATION: GLASS BEADS AS AN INNOVATIVE MODEL CARRIER SYSTEM DOCTORAL THESIS SUMBITTED IN FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR IN NATURAL SCIENCES AT KIEL UNIVERSITY, GERMANY by Niklas Ludwig Renner Kiel 2017 Referee: Prof. Dr. Regina Scherließ Co-Referee: Prof. Dr. Thomas Kunze Date of Exam: 13.10.2017 Accepted for publication: 13.10.2017 Prof. Dr. Natascha Oppelt (Dean) Published research articles contributing to the present thesis: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler performance Dalby, R.N. (Ed.), RDD Europe 2017 2 (2017), 195-200 Renner, N; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Nano- and microstructured model carrier surfaces to alter dry powder inhaler performance International Journal of Pharmaceutics 518 (2017), 20-28 Conference contributions: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler per- formance Respiratory Drug Delivery, Nice, France (2017) Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. A deeper insight into the impact of chemical surface properties on inhalation perfor- mance Drug Delivery to the Lungs 27, Edinburgh, Scotland (2016) Renner, N.; Scherließ, R.; Steckel, H. Glass beads as model carriers in dry powder inhalers: the influence of chemical sur- face properties on inhalation performance International Congress on Particle Technology, Nurnberg, Germany (2016) Renner, N.; Scherließ, R.; Steckel, H. Investigating the influence of carrier surface roughness on drug delivery in DPIs 10th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Tech- nology, Glasgow, Scotland (2016) Renner, N.; Kutelova, Z.; Scherließ, R.; Steckel, H. -

The Role of 5-Lipoxygenase in Pathophysiology and Management of Neuropathic Pain
REVIEW ARTICLE THE ROLE OF 5-LIPOXYGENASE IN PATHOPHYSIOLOGY AND MANAGEMENT OF NEUROPATHIC PAIN Pascanus Lamsihar PT∗, Faldi Yaputra∗∗, Jimmy FA Barus4 and I Putu Eka Widyadharma∗∗,1 ∗Department of Neurology, Provincial Mental Hospital, West Borneo, Indonesia., ∗∗Department of Neurology, Faculty of Medicine, Udayana University-Sanglah General Hospital, Bali, Indonesia., 4Department of Neurology, Faculty of Medicine, Atma Jaya Catholic University, Jakarta-Indonesia. ABSTRACT Neuropathic pain (NP) is a pain caused by lesions in the nervous system. Several causes of NP are traumatic, metabolic disorders, ischemia, toxins, infections, immune-related, and hereditary. The pathophysiology of NP is very complicated and unknown entirely. Therefore the treatment of NP is still unsatisfactory. Recent studies believed the critical role of primary inflammatory mediators in the pathophysiology of NP especially leukotrienes (LTs). The 5- lipoxygenase enzyme (5-LOX) is an enzyme that plays a role in the metabolism of arachidonic acid into LTs. Leukotrienes (LTs) are the essential inflammatory mediators in the pathophysiology of NP. Leukotriene B4 (LTB4) can cause chemotaxis on neutrophils, lowering nociceptors threshold and may contribute to NP. Several studies believed the administration of 5-LOX inhibitors or LTs receptor antagonists could be useful in the management of NP. The purpose of this review is to summarize the involvement of 5-LOX enzyme as an essential role in the pathophysiology and management of NP. KEYWORDS 5-lipoxygenase, leukotrienes, -

Intracolonic Administration of Zileuton, a Selective 5-Lipoxygenase Inhibitor, Accelerates Healing in a Rat Model of Chronic Colitis
Gut 1996; 38: 899-904 899 Intracolonic administration of zileuton, a selective 5-lipoxygenase inhibitor, accelerates healing in a rat model of chronic colitis Gut: first published as 10.1136/gut.38.6.899 on 1 June 1996. Downloaded from X Bertran, J Mafe', F Fernandez-Bafiares, E Castella, R Bartoli, I Ojanguren, M Esteve, M A Gassull Abstract models of colitis that the concentration of Background-5-Lipoxygenase products leukotrienes (mainly leukotriene B4 (LTB4)) in play a part in inflammatory response. the inflamed mucosa is within the range known Aims-The effect of intracolonic admini- to induce biological effects (chemokinesis, cell stration of zileuton (a 5-lipoxygenase aggregation, increasing of vascular permeabil- inhibitor) on colonic damage and ity), and these concentrations are similar to eicosanoid local release was assessed in a those seen in human IBD.2-7 In addition, rat model ofcolitis. drugs known to be effective in the treatment of Methods-Ninety rats with trinitroben- IBD are capable of reducing intestinal zenesulphonic acid induced colitis were leukotriene production in both experimental randomised to receive placebo, 5-amino- models of colitis and human IBD.36 On the salicylic acid (50 mglkg), or zileuton other hand, administration ofpotent and selec- (50 mg/kg) intracolonically for four weeks. tive inhibitors of leukotriene synthesis results Local eicosanoid release was monitored in a reduction of macroscopic and histological by intracolonic dialysis throughout the colonic damage in experimental colitis.6 8-11 study. The colon was removed for macro- Zileuton, N-(1-(benzo[b]thien-2-yl)ethyl)- scopic and histological assessment at N-hydroxyurea (Abbott Laboratories, Abbott weeks 1, 2, and 4 after colitis induction in Park, IL), is a drug that selectively inhibits the 10 rats of each group. -

The Role of Pharmacogenomics in Improving the Management of Asthma
The Role of Pharmacogenomics in Improving the Management of Asthma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kazani S, ME Wechsler, E. Israel. 2010. TThe Role of Pharmacogenomics in Improving the Management of Asthma. Journal of Allergy and Clinical Immunology 125, no. 2: 295–302. Published Version 10.1016/j.jaci.2009.12.014 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42659246 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP NIH Public Access Author Manuscript J Allergy Clin Immunol. Author manuscript; available in PMC 2011 February 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Allergy Clin Immunol. 2010 February ; 125(2): 295±302. doi:10.1016/j.jaci.2009.12.014. THE ROLE OF PHARMACOGENOMICS IN IMPROVING THE MANAGEMENT OF ASTHMA Shamsah Kazani, M.D. [Instructor in Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Michael E. Wechsler, M.D. [Assistant Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Elliot Israel, M.D. [Associate Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Abstract There is a large amount of interindividual variability in both therapeutic and adverse responses to asthma therapies. Genetic variability may account for 50–60% of this variability.