The Role of 5-Lipoxygenase in Pathophysiology and Management of Neuropathic Pain
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

1Β IL-12 Receptor Viral Inflammation Are Mediated Through Macrophage
IL-12 p40 Homodimer-Dependent Macrophage Chemotaxis and Respiratory Viral Inflammation Are Mediated through IL-12 Receptor β1 This information is current as of September 24, 2021. Tonya D. Russell, Qingyun Yan, Guangshun Fan, Anthony P. Khalifah, D. Keith Bishop, Steven L. Brody and Michael J. Walter J Immunol 2003; 171:6866-6874; ; doi: 10.4049/jimmunol.171.12.6866 Downloaded from http://www.jimmunol.org/content/171/12/6866 References This article cites 63 articles, 41 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/171/12/6866.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 24, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2003 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology IL-12 p40 Homodimer-Dependent Macrophage Chemotaxis and Respiratory Viral Inflammation Are Mediated through IL-12 Receptor 11 Tonya D. Russell,* Qingyun Yan,* Guangshun Fan,* Anthony P. -

Drug Information Sheet("Kusuri-No-Shiori")
Drug Information Sheet("Kusuri-no-Shiori") Internal Published: 05/2017 The information on this sheet is based on approvals granted by the Japanese regulatory authority. Approval details may vary by country. Medicines have adverse reactions (risks) as well as efficacies (benefits). It is important to minimize adverse reactions and maximize efficacy. To obtain a better therapeutic response, patients should understand their medication and cooperate with the treatment. Brand name:PRANLUKAST TABLETS 112.5mg "CEO" Active ingredient:Pranlukast hydrate Dosage form:white to pale yellow tablet, diameter: 7.5 mm, thickness: 2.7 mm Print on wrapping:(face) プランルカスト 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg (back) PRANLUKAST 112.5mg「CEO」, CEO 131, プランルカスト 112.5mg Effects of this medicine This medicine selectively binds to leukotriene receptor and inhibits its action. It consequently suppresses increase in airway contraction, vascular permeability, mucosal edema and hypersensitivity. It is usually used to treat bronchial asthma and allergic rhinitis. However, it cannot stop the attack of bronchial asthma already in progress but prevents the asthma attack. Before using this medicine, be sure to tell your doctor and pharmacist ・If you have previously experienced any allergic reactions (itch, rash, etc.) to any medicines. ・If you are pregnant or breastfeeding. ・If you are taking any other medicinal products. (Some medicines may interact to enhance or diminish medicinal effects. Beware of over-the-counter medicines and dietary supplements as well as other prescription medicines.) Dosing schedule (How to take this medicine) ・Your dosing schedule prescribed by your doctor is(( to be written by a healthcare professional)) ・In general, for adults, take 2 tablets (225 mg of the active ingredient) at a time, twice a day after breakfast and dinner. -

Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Showa Univ J Med Sci 21(2), 77~84, June 2009 Original Inhibitory Activity of Pranlukast and Montelukas Against Histamine Release and LTC4 Production from Human Basophils 1, 2 1 1 Satoshi HIBINO ), Ryoko ITO ), Taeru KITABAYASHI ), 1 2 Kazuo ITAHASHI ) and Toshio NAKADATE ) Abstract : Leukotriene receptor antagonists(LTRAs)are routinely used to treat bronchial asthma and are thought to act mostly by inhibiting leukotriene receptors. However, there is no preclinical or clinical evidence of the direct effect of LTRAs on histamine release from and leukotriene(LT)C4 produc- tion by basophils. We used anti-IgE antibody(Ab), FMLP, and C5a to induce histamine release, and anti-IgE Ab and FMLP to stimulate LTC 4 production. Basophils were exposed to different concentrations of pranlukast and montelu- kast, and then to anti-IgE Ab, FMLP, and C5a. Culture supernatant histamine and LTC 4 levels were measured by using a histamine ELISA kit and a LTC 4 EIA kit, respectively. Histamine release was expressed as a percentage of the total histamine content(%HR)induced by anti-IgE Ab, FMLP, or C5a. To evaluate the effects of pranlukast and montelukast on histamine release and LTC 4 production, we calculated the percent inhibition of histamine release and LTC 4 production, expressed as percent inhibition, at different concentrations of pranlukast and montelukast. Pranlukast significantly inhibited histamine release stimulated by FMLP and C5a, but had no effect on histamine release stimulated by anti-IgE Ab. By comparison, montelukast signicantly inhibited histamine release stimulated by FMLP, C5a, and anti-IgE Ab, in a concentration-dependent manner. Both pranlukast and montelukast signicantly inhibited LTC 4 production stimulated by anti-IgE Ab and FMLP. -

Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid
Comprehensive Summaries of Uppsala Dissertations from the Faculty of Pharmacy 231 _____________________________ _____________________________ Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21 BY JOHAN BYLUND ACTA UNIVERSITATIS UPSALIENSIS UPPSALA 2000 Dissertation for the Degree of Doctor of Philosophy (Faculty of Pharmacy) in Pharmaceutical Pharmacology presented at Uppsala University in 2000 ABSTRACT Bylund, J. 2000. Cytochrome P450 Enzymes in Oxygenation of Prostaglandin Endoperoxides and Arachidonic Acid: Cloning, Expression and Catalytic Properties of CYP4F8 and CYP4F21. Acta Universitatis Upsaliensis. Comprehensive Summaries of Uppsala Dissertations from Faculty of Pharmacy 231 50 pp. Uppsala. ISBN 91-554-4784-8. Cytochrome P450 (P450 or CYP) is an enzyme system involved in the oxygenation of a wide range of endogenous compounds as well as foreign chemicals and drugs. This thesis describes investigations of P450-catalyzed oxygenation of prostaglandins, linoleic and arachidonic acids. The formation of bisallylic hydroxy metabolites of linoleic and arachidonic acids was studied with human recombinant P450s and with human liver microsomes. Several P450 enzymes catalyzed the formation of bisallylic hydroxy metabolites. Inhibition studies and stereochemical analysis of metabolites suggest that the enzyme CYP1A2 may contribute to the biosynthesis of bisallylic hydroxy fatty acid metabolites in adult human liver microsomes. 19R-Hydroxy-PGE and 20-hydroxy-PGE are major components of human and ovine semen, respectively. They are formed in the seminal vesicles, but the mechanism of their biosynthesis is unknown. Reverse transcription-polymerase chain reaction using degenerate primers for mammalian CYP4 family genes, revealed expression of two novel P450 genes in human and ovine seminal vesicles. -

Eicosanoids Mediate Insect Nodulation Responses to Bacterial Infections (Cyclooxygenase/Lipoxygenase/Phospholipase A2/Manduca Sexta/Serratia Marcescens) JON S
Proc. Natl. Acad. Sci. USA Vol. 91, pp. 12418-12422, December 1994 Physiology Eicosanoids mediate insect nodulation responses to bacterial infections (cyclooxygenase/lipoxygenase/phospholipase A2/Manduca sexta/Serratia marcescens) JON S. MILLER, TUANH NGUYEN, AND DAVID W. STANLEY-SAMUELSON* Insect Biochemistry/Physiology Laboratory, Department of Entomology, University of Nebraska, Lincoln, NE 68583-0816 Communicated by Wendell L. Roelofs, September 12, 1994 (receivedfor review August 11, 1993) ABSTRACT We propose that nodule formation is medi- inhibiting eicosanoid biosynthesis. On the basis of these ated by eicosanoids in insects. Nodulation is the temporally and findings, we proposed that eicosanoid products of the cyclo- quantitatively predominant cellular defense response to bac- oxygenase and lipoxygenase pathways are involved in insect terial infection in insects and other invertebrates. Inhibition of immune responses to bacterial infections. Because most of eicosanoid biosynthesis in larvae of the tobacco hornworn our experiments were done during the first hour postinfection Manduca sexta immediately prior to intrahemocoelic infections (PI), long before the appearance of antibacterial proteins in with the bacterium Serratia marcescens strongly reduced the insect hemolymph, we suggested that eicosanoids mediate nodulation response. Inhibition of eicosanoid biosynthesis also one or more hemocytic defense responses (6). reduced formation of cellular aggregates at 1 hr postinfection, This suggestion opens a crucial question: which -

Engineering 3D Systems with Tunable Mechanical Properties to Mimic the Tumor Microenvironment Shalini Raj Unnikandam Veettil Iowa State University
Iowa State University Capstones, Theses and Graduate Theses and Dissertations Dissertations 2018 Engineering 3D systems with tunable mechanical properties to mimic the tumor microenvironment Shalini Raj Unnikandam Veettil Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/etd Part of the Chemical Engineering Commons Recommended Citation Unnikandam Veettil, Shalini Raj, "Engineering 3D systems with tunable mechanical properties to mimic the tumor microenvironment" (2018). Graduate Theses and Dissertations. 17339. https://lib.dr.iastate.edu/etd/17339 This Thesis is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Graduate Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. Engineering 3D systems with tunable mechanical properties to mimic the tumor microenvironment by Shalini Raj Unnikandam Veettil A thesis submitted to the graduate faculty in partial fulfillment of the requirements for the degree of MASTER OF SCIENCE Major: Chemical Engineering Program of Study Committee: Ian C Schneider, Major Professor Kaitlin Bratlie Michael Bartlett The student author, whose presentation of the scholarship herein was approved by the program of study committee, is solely responsible for the content of this thesis. The Graduate College will ensure this thesis is globally accessible and will not permit alterations after a degree is conferred. Iowa State University Ames, Iowa 2018 Copyright © Shalini Raj Unnikandam Veettil, 2018. All rights reserved. ii DEDICATION This thesis is dedicated to my family and friends who have been a great source of support. -

Drug Class Review Controller Medications for Asthma
Drug Class Review Controller Medications for Asthma Final Update 1 Report April 2011 The Agency for Healthcare Research and Quality has not yet seen or approved this report. The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Original Report: November 2008 Daniel E. Jonas, MD, MPH Roberta C. M. Wines, MPH Marcy DelMonte, PharmD, BCPS Halle R. Amick, MSPH Tania M. Wilkins, MS Brett D. Einerson, MPH Christine L. Schuler, MD Blake A. Wynia, MPH Betsy Bryant Shilliday, Pharm.D., CDE, CPP Produced by RTI-UNC Evidence-based Practice Center Cecil G. Sheps Center for Health Services Research University of North Carolina at Chapel Hill 725 Martin Luther King Jr. Blvd, CB# 7590 Chapel Hill, NC 27599-7590 Tim Carey, M.D., M.P.H., Director Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Copyright © 2011 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Update 1 Report Drug Effectiveness Review Project The medical literature relating to this topic is scanned periodically. (See http://www.ohsu.edu/xd/research/centers-institutes/evidence-based-policy- center/derp/documents/methods.cfm for description of scanning process). Prior versions of this report can be accessed at the DERP website. -

Lipoxygenase Inhibition Activity of Coumarin Derivatives—QSAR and Molecular Docking Study
pharmaceuticals Article Lipoxygenase Inhibition Activity of Coumarin Derivatives—QSAR and Molecular Docking Study Melita Lonˇcari´c 1 , Ivica Strelec 1 , Valentina Pavi´c 2 , Domagoj Šubari´c 3, Vesna Rastija 3 and Maja Molnar 1,* 1 Faculty of Food Faculty Osijek, Josip Juraj Strossmayer University, 31000 Osijek, Croatia; [email protected] (M.L.); [email protected] (I.S.) 2 Department of Biology, Josip Juraj Strossmayer University of Osijek, 31000 Osijek, Croatia; [email protected] 3 Department of Agroecology and Environmental Protection, Faculty of Agrobiotechnical Sciences Osijek, Josip Juraj Strossmayer University of Osijek, 31000 Osijek, Croatia; [email protected] (D.Š.); [email protected] (V.R.) * Correspondence: [email protected]; Tel.: +385-31-224-342 Received: 2 July 2020; Accepted: 15 July 2020; Published: 17 July 2020 Abstract: Lipoxygenases (LOXs) are a family of enzymes found in plants, mammals, and microorganisms. In animals and plants, the enzyme has the capability for the peroxidation of unsaturated fatty acids. Although LOXs participate in the plant defense system, the enzyme’s metabolites can have numerous negative effects on human health. Therefore, many types of research are searching for compounds that can inhibit LOXs. The best quantitative structure–activity relationship (QSAR) model was obtained using a Genetic Algorithm (GA). Molecular docking was performed with iGEMDOCK. The inhibition of lipoxygenase was in the range of 7.1 to 96.6%, and the inhibition of lipid peroxidation was 7.0–91.0%. Among the synthesized compounds, the strongest inhibitor of soybean LOX-3 (96.6%) was found to be 3-benzoyl- 7-(benzyloxy)-2H-chromen-2-one. -

Understanding Interparticle Interactions in Dry Powder Inhalation: Glass Beads As an Innovative Model Carrier System
UNDERSTANDING INTERPARTICLE INTERACTIONS IN DRY POWDER INHALATION: GLASS BEADS AS AN INNOVATIVE MODEL CARRIER SYSTEM DOCTORAL THESIS SUMBITTED IN FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR IN NATURAL SCIENCES AT KIEL UNIVERSITY, GERMANY by Niklas Ludwig Renner Kiel 2017 Referee: Prof. Dr. Regina Scherließ Co-Referee: Prof. Dr. Thomas Kunze Date of Exam: 13.10.2017 Accepted for publication: 13.10.2017 Prof. Dr. Natascha Oppelt (Dean) Published research articles contributing to the present thesis: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler performance Dalby, R.N. (Ed.), RDD Europe 2017 2 (2017), 195-200 Renner, N; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Nano- and microstructured model carrier surfaces to alter dry powder inhaler performance International Journal of Pharmaceutics 518 (2017), 20-28 Conference contributions: Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. Tailoring the surface topography of a model carrier to alter dry powder inhaler per- formance Respiratory Drug Delivery, Nice, France (2017) Renner, N.; Steckel, H.; Urbanetz, N.A.; Scherließ, R. A deeper insight into the impact of chemical surface properties on inhalation perfor- mance Drug Delivery to the Lungs 27, Edinburgh, Scotland (2016) Renner, N.; Scherließ, R.; Steckel, H. Glass beads as model carriers in dry powder inhalers: the influence of chemical sur- face properties on inhalation performance International Congress on Particle Technology, Nurnberg, Germany (2016) Renner, N.; Scherließ, R.; Steckel, H. Investigating the influence of carrier surface roughness on drug delivery in DPIs 10th World Meeting on Pharmaceutics, Biopharmaceutics and Pharmaceutical Tech- nology, Glasgow, Scotland (2016) Renner, N.; Kutelova, Z.; Scherließ, R.; Steckel, H. -

The Role of Pharmacogenomics in Improving the Management of Asthma
The Role of Pharmacogenomics in Improving the Management of Asthma The Harvard community has made this article openly available. Please share how this access benefits you. Your story matters Citation Kazani S, ME Wechsler, E. Israel. 2010. TThe Role of Pharmacogenomics in Improving the Management of Asthma. Journal of Allergy and Clinical Immunology 125, no. 2: 295–302. Published Version 10.1016/j.jaci.2009.12.014 Citable link http://nrs.harvard.edu/urn-3:HUL.InstRepos:42659246 Terms of Use This article was downloaded from Harvard University’s DASH repository, and is made available under the terms and conditions applicable to Open Access Policy Articles, as set forth at http:// nrs.harvard.edu/urn-3:HUL.InstRepos:dash.current.terms-of- use#OAP NIH Public Access Author Manuscript J Allergy Clin Immunol. Author manuscript; available in PMC 2011 February 1. NIH-PA Author ManuscriptPublished NIH-PA Author Manuscript in final edited NIH-PA Author Manuscript form as: J Allergy Clin Immunol. 2010 February ; 125(2): 295±302. doi:10.1016/j.jaci.2009.12.014. THE ROLE OF PHARMACOGENOMICS IN IMPROVING THE MANAGEMENT OF ASTHMA Shamsah Kazani, M.D. [Instructor in Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Michael E. Wechsler, M.D. [Assistant Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Elliot Israel, M.D. [Associate Professor of Medicine] Harvard Medical School Brigham and Women's Hospital Boston, Massachusetts Abstract There is a large amount of interindividual variability in both therapeutic and adverse responses to asthma therapies. Genetic variability may account for 50–60% of this variability. -

DMD # 35121 1 Human Cytochrome P450, CYP2S1, Metabolizes
DMD Fast Forward. Published on November 10, 2010 as DOI: 10.1124/dmd.110.035121 DMD FastThis Forward. article has not Published been copyedited on andNovember formatted. The 10, final 2010 version as maydoi:10.1124/dmd.110.035121 differ from this version. DMD # 35121 Human Cytochrome P450, CYP2S1, Metabolizes Cyclooxygenase – and Lipoxygenase – Derived Eicosanoids Peter Bui, Satoshi Imaizumi, Sudheer Reddy Beedanagari, Srinivasa T. Reddy, and Oliver Hankinson Department of Pathology and Laboratory Medicine (P.B., S.R.B., and O.H.), Molecular Toxicology Interdepartmental Program (P.B., S.R.B., and O.H.) , Jonsson Comprehensive Cancer Center (P.B., S.R.B., S.T.R., and O.H.), Department of Medicine (S.I., and S.T.R.), and Downloaded from Department of Molecular and Medical Pharmacology (S.T.R.), University of California at Los Angeles, Los Angeles, California 90095 dmd.aspetjournals.org at ASPET Journals on September 26, 2021 1 Copyright 2010 by the American Society for Pharmacology and Experimental Therapeutics. DMD Fast Forward. Published on November 10, 2010 as DOI: 10.1124/dmd.110.035121 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 35121 Running title: CYP2S1 metabolizes eicosanoids To whom correspondence should be addressed: Oliver Hankinson, Ph.D. Departmental of Pathology and Laboratory Medicine, David Geffen School of Medicine, University of California at Los Angeles. 650 Charles E. Young Drive, Los Angeles, CA. 90095 Tel: 310-825-2936 Fax: 310-794-9272 Email: [email protected] Downloaded from Text: 36 pages Tables: 4 dmd.aspetjournals.org Figures: 10 References: 44 Abstract: 228 words at ASPET Journals on September 26, 2021 Introduction: 588 words Discussion: 1,146 words Abbreviations: CYP and P450, cytochrome P450; HPLC, high-performance liquid chromatography; LC-ESI-MS, liquid chromatography-electrospray ionization-mass spectrometry; MS/MS, tandem mass spectrometry; m/z, mass-to-charge ratio; M-H, molecular mass minus 1 2 DMD Fast Forward. -
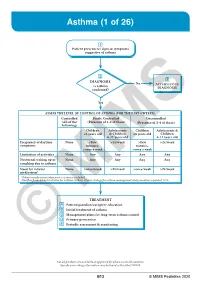
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough