Monteleucast and Zileuton Retard the Progression of Atherosclerosis Via Down Regulation of the Inflammatory and Oxidative Pathwa
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Inhibitory Activity of Pranlukast and Montelukas Against Histamine
Showa Univ J Med Sci 21(2), 77~84, June 2009 Original Inhibitory Activity of Pranlukast and Montelukas Against Histamine Release and LTC4 Production from Human Basophils 1, 2 1 1 Satoshi HIBINO ), Ryoko ITO ), Taeru KITABAYASHI ), 1 2 Kazuo ITAHASHI ) and Toshio NAKADATE ) Abstract : Leukotriene receptor antagonists(LTRAs)are routinely used to treat bronchial asthma and are thought to act mostly by inhibiting leukotriene receptors. However, there is no preclinical or clinical evidence of the direct effect of LTRAs on histamine release from and leukotriene(LT)C4 produc- tion by basophils. We used anti-IgE antibody(Ab), FMLP, and C5a to induce histamine release, and anti-IgE Ab and FMLP to stimulate LTC 4 production. Basophils were exposed to different concentrations of pranlukast and montelu- kast, and then to anti-IgE Ab, FMLP, and C5a. Culture supernatant histamine and LTC 4 levels were measured by using a histamine ELISA kit and a LTC 4 EIA kit, respectively. Histamine release was expressed as a percentage of the total histamine content(%HR)induced by anti-IgE Ab, FMLP, or C5a. To evaluate the effects of pranlukast and montelukast on histamine release and LTC 4 production, we calculated the percent inhibition of histamine release and LTC 4 production, expressed as percent inhibition, at different concentrations of pranlukast and montelukast. Pranlukast significantly inhibited histamine release stimulated by FMLP and C5a, but had no effect on histamine release stimulated by anti-IgE Ab. By comparison, montelukast signicantly inhibited histamine release stimulated by FMLP, C5a, and anti-IgE Ab, in a concentration-dependent manner. Both pranlukast and montelukast signicantly inhibited LTC 4 production stimulated by anti-IgE Ab and FMLP. -
Medication Coverage Policy
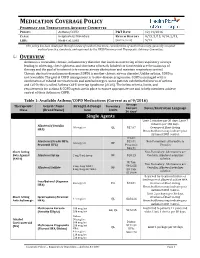
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2016 CLASS: Respiratory Disorders REVIEW HISTORY 9/15, 5/15, 9/14, 2/13, LOB: Medi-Cal, SJHA (MONTH/YEAR) 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee. OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 9/2016) Average Therapeutic Generic Name Strength & Dosage Formulary Cost per Limits Notes/Restriction Language Class (Brand Name) form 30 days* Single Agents Limit 2 inhalers per 30 days; Limit 7 -

Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma
Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma Color Key n Four Components of Asthma Care n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 0– 4 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 5–11 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children >12 Years of Age & Adults n Long-Term Control Medications: Estimated Comparative Daily Dosages n Long-Term Control Medications: Usual Dosages n Quick-Relief Medications Guidelines are intended to be flexible. They serve as recommendations, not rigid criteria. Guidelines should be followed in most cases, but depending on the patient, and the circumstances, guidelines may need to be tailored to fit individual needs. 4750 11/18 Contents Criteria that suggest the diagnosis of asthma . 3 Goal of Therapy: Control of Asthma . 3 Four Components of Asthma Care 1. Assessment and Monitoring of Asthma Severity and Control . 4 2. Education for a Partnership in Care . 5 3. Control of Environmental Factors and Co-morbid Conditions that Affect Asthma . 5 4. Medications . 6 Bibliography . 6 Classifying Asthma Severity & Initiating Treatment in Children 0– 4 Years of Age . 7 Assessing Asthma Control & Adjusting Therapy in Children 0– 4 Years of Age . 7 Stepwise Approach for Managing Asthma in Children 0– 4 Years of Age . 8 Classifying Asthma Severity & Initiating Treatment in Children 5–11 Years of Age . 9 Assessing Asthma Control & Adjusting Therapy in Children 5–11 Years of Age . -

Montreal Protocol on Substances That Deplete the Ozone Layer
MONTREAL PROTOCOL ON SUBSTANCES THAT DEPLETE THE OZONE LAYER UNEP 2006 REPORT OF THE MEDICAL TECHNICAL OPTIONS COMMITTEE 2006 ASSESSMENT UNEP 2006 REPORT OF THE MEDICAL TECHNICAL OPTIONS COMMITTEE 2006 ASSESSMENT 2006 MTOC Assessment Report iii Montreal Protocol On Substances that Deplete the Ozone Layer Report of the UNEP Medical Technical Options Committee 2006 Assessment ASSESSMENT REPORT The text of this report is composed in Times New Roman. Co-ordination: Medical Technical Options Committee Composition and layout: Helen Tope Reproduction: UNON Nairobi Date: January 2007 Under certain conditions, printed copies of this report are available from: UNITED NATIONS ENVIRONMENT PROGRAMME Ozone Secretariat, P.O. Box 30552, Nairobi, Kenya This document is also available in portable document format from the UNEP Ozone Secretariat's website: http://ozone.unep.org/Assessment_Panels/TEAP/Reports/MTOC/ No copyright involved. This publication may be freely copied, abstracted and cited, with acknowledgement of the source of the material. ISBN: 978-92-807-2828-6 Job No: OZO/0954/NA Cover photograph © Luo Hong. iv 2006 MTOC Assessment Report Disclaimer The United Nations Environment Programme (UNEP), the Technology and Economic Assessment Panel (TEAP) Co-chairs and members, the Technical Options Committees Co-chairs and members, the TEAP Task Forces Co-chairs and members, and the companies and organisations that employ them do not endorse the performance, worker safety, or environmental acceptability of any of the technical options discussed. Every industrial operation requires consideration of worker safety and proper disposal of contaminants and waste products. Moreover, as work continues - including additional toxicity evaluation - more information on health, environmental and safety effects of alternatives and replacements will become available for use in selecting among the options discussed in this document. -

Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity
ID Design Press, Skopje, Republic of Macedonia Open Access Macedonian Journal of Medical Sciences. 2018 May 20; 6(5):777-781. https://doi.org/10.3889/oamjms.2018.187 eISSN: 1857-9655 Clinical Science Comparison of Effect of Leukotriene Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial Hyperreactivity Naim Morina1, Arsim Haliti1, Ali Iljazi2, Drita Islami3, Sadi Bexheti4, Adnan Bozalija1*, Hilmi Islami3 1Department of Pharmacy, Faculty of Medicine, University of Prishtina, Prishtina, Kosovo; 2Kosovo Occupational Health Institute, Gjakovo, Kosovo; 3Department of Pharmacology, Faculty of Medicine, University of Prishtina, Kosovo; 4Department of Anatomy, Faculty of Medicine, University of Prishtina, Kosovo Abstract Citation: Morina N, Haliti A, Iljazi A, Islami D, Bexheti S, AIM: Blocking effect of leukotriene biosynthesis–zileuton and blocking the effect of phosphodiesterase enzyme– Bozalija A, Islami H. Comparison of Effect of Leukotriene diprophylline in the treatment of patients with bronchial asthma and bronchial increased reactivity, and tiotropium Biosynthesis Blockers and Inhibitors of Phosphodiesterase Enzyme in Patients with Bronchial bromide as an antagonist of the muscarinic receptor studied in this work. Hyperreactivity. Open Access Maced J Med Sci. 2018 May 20; 6(5):777-781. METHODS: Parameters of the lung function are determined with Body plethysmography. The resistance of the https://doi.org/10.3889/oamjms.2018.187 airways (Raw) was registered and measured was intrathoracic gas volume (ITGV), and specific resistance Keywords: Bronchial asthma; Zileuton; Diprophylline and tiotropium bromide (SRaw) was also calculated. For the research, administered was zileuton (tabl. 600 mg) and diprophylline (tabl. 150 mg). *Correspondence: Adnan Bozalija. Department of Pharmacy, Faculty of Medicine, University of Prishtina, Prishtina, Kosovo. -

Montelukast Sodium) Tablets, Chewable Tablets, and Oral
Merck Sharp & Dohme Corp., a subsidiary of o MERCK & CO., INC. Whitehouse Station, NJ 08889/ USA HIGHLIGHTS OF PRESCRIBING INFORMATION .....................DOSAGE FORMS AND STRENGTHS..................... These highlights do not include all the information needed to use . SINGULAIR 10-mg Film-Coated Tablets SINGULAIR safely and effectively. See full prescribing information . SINGULAIR 5-mg and 4-mg Chewable Tablets for SINGULAIR. SINGULAIR 4.mg Oral Granules SINGULAIRiI .............................. CONTRAINDICATIONS .............................. (montelukast sodium) tablets, chewable tablets, and oral . Hypersensitivity to any component of this product (4). granules ....................... WARNINGS AND PRECAUTIONS ....................... Initial U.S. Approval: 1998 . Do not prescribe SINGULAIR to treat an acute asthma attack. .......................... RECENT MAJOR CHANGES........................... Advise patients to have appropriate rescue medication available Warnings and Precautions, Neuropsychiatric Events (5.4) 04/2010 (5.1). Inhaled corticosteroid may be reduced gradually. Do not abruptly ...........................INDICATIONS AND USAGE ........................... substitute SINGULAIR for inhaled or oral corticosteroids (5.2). SINGULAIRiI is a leukotriene receptor antagonist indicated for: . Patients with known aspirin sensitivity should continue to avoid . Prophylaxis and chronic treatment of asthma in patients aspirin or non-steroidal anti-inflammatory agents while taking 12 months of age and older (1.1). SINGULAIR (5.3). Acute prevention of exercise-induced bronchoconstriction (EIS) in Neuropsychiatric events have been reported with SINGULAIR. patients 15 years of age and older (1.2). Instruct patients to be alert for neuropsychiatric events. Evaluate Relief of symptoms of allergic rhinitis (AR): seasonal allergic the risks and benefits of continuing treatment with SINGULAIR if rhinitis (SAR) in patients 2 years of age and older, and perennial such events occur (5.4 and 6.2). -

FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting
FDA Briefing Document Pulmonary-Allergy Drugs Advisory Committee Meeting August 31, 2020 sNDA 209482: fluticasone furoate/umeclidinium/vilanterol fixed dose combination to reduce all-cause mortality in patients with chronic obstructive pulmonary disease NDA209482/S-0008 PADAC Clinical and Statistical Briefing Document Fluticasone furoate/umeclidinium/vilanterol fixed dose combination for all-cause mortality DISCLAIMER STATEMENT The attached package contains background information prepared by the Food and Drug Administration (FDA) for the panel members of the advisory committee. The FDA background package often contains assessments and/or conclusions and recommendations written by individual FDA reviewers. Such conclusions and recommendations do not necessarily represent the final position of the individual reviewers, nor do they necessarily represent the final position of the Review Division or Office. We have brought the supplemental New Drug Application (sNDA) 209482, for fluticasone furoate/umeclidinium/vilanterol, as an inhaled fixed dose combination, for the reduction in all-cause mortality in patients with COPD, to this Advisory Committee in order to gain the Committee’s insights and opinions, and the background package may not include all issues relevant to the final regulatory recommendation and instead is intended to focus on issues identified by the Agency for discussion by the advisory committee. The FDA will not issue a final determination on the issues at hand until input from the advisory committee process has been considered -

Intracolonic Administration of Zileuton, a Selective 5-Lipoxygenase Inhibitor, Accelerates Healing in a Rat Model of Chronic Colitis
Gut 1996; 38: 899-904 899 Intracolonic administration of zileuton, a selective 5-lipoxygenase inhibitor, accelerates healing in a rat model of chronic colitis Gut: first published as 10.1136/gut.38.6.899 on 1 June 1996. Downloaded from X Bertran, J Mafe', F Fernandez-Bafiares, E Castella, R Bartoli, I Ojanguren, M Esteve, M A Gassull Abstract models of colitis that the concentration of Background-5-Lipoxygenase products leukotrienes (mainly leukotriene B4 (LTB4)) in play a part in inflammatory response. the inflamed mucosa is within the range known Aims-The effect of intracolonic admini- to induce biological effects (chemokinesis, cell stration of zileuton (a 5-lipoxygenase aggregation, increasing of vascular permeabil- inhibitor) on colonic damage and ity), and these concentrations are similar to eicosanoid local release was assessed in a those seen in human IBD.2-7 In addition, rat model ofcolitis. drugs known to be effective in the treatment of Methods-Ninety rats with trinitroben- IBD are capable of reducing intestinal zenesulphonic acid induced colitis were leukotriene production in both experimental randomised to receive placebo, 5-amino- models of colitis and human IBD.36 On the salicylic acid (50 mglkg), or zileuton other hand, administration ofpotent and selec- (50 mg/kg) intracolonically for four weeks. tive inhibitors of leukotriene synthesis results Local eicosanoid release was monitored in a reduction of macroscopic and histological by intracolonic dialysis throughout the colonic damage in experimental colitis.6 8-11 study. The colon was removed for macro- Zileuton, N-(1-(benzo[b]thien-2-yl)ethyl)- scopic and histological assessment at N-hydroxyurea (Abbott Laboratories, Abbott weeks 1, 2, and 4 after colitis induction in Park, IL), is a drug that selectively inhibits the 10 rats of each group. -

Singulair (Montelukast Sodium)
HIGHLIGHTS OF PRESCRIBING INFORMATION These highlights do not include all the information needed to use --------------------- DOSAGE FORMS AND STRENGTHS -------------------- SINGULAIR safely and effectively. See full prescribing information SINGULAIR 10-mg Film-Coated Tablets for SINGULAIR. SINGULAIR 5-mg and 4-mg Chewable Tablets SINGULAIR 4-mg Oral Granules (3) SINGULAIR® (montelukast sodium) Tablets, Chewable Tablets, -------------------------------CONTRAINDICATIONS ------------------------------ and Oral Granules Hypersensitivity to any component of this product (4). Initial U.S. Approval: 1998 ------------------------WARNINGS AND PRECAUTIONS----------------------- ---------------------------RECENT MAJOR CHANGES -------------------------- Do not prescribe SINGULAIR to treat an acute asthma attack Indications and Usage (5.1). Exercise-Induced Bronchoconstriction (EIB) (1.2) 03/2012 Dosage and Administration Advise patients to have appropriate rescue medication available Exercise-Induced Bronchoconstriction (EIB) (2.2) 03/2012 (5.1). Inhaled corticosteroid may be reduced gradually. Do not abruptly ----------------------------INDICATIONS AND USAGE --------------------------- substitute SINGULAIR for inhaled or oral corticosteroids (5.2). SINGULAIR is a leukotriene receptor antagonist indicated for: Patients with known aspirin sensitivity should continue to avoid Prophylaxis and chronic treatment of asthma in patients aspirin or non-steroidal anti-inflammatory agents while taking 12 months of age and older (1.1). SINGULAIR (5.3). Acute prevention of exercise-induced bronchoconstriction (EIB) in Neuropsychiatric events have been reported with SINGULAIR. patients 6 years of age and older (1.2). Instruct patients to be alert for neuropsychiatric events. Evaluate Relief of symptoms of allergic rhinitis (AR): seasonal allergic the risks and benefits of continuing treatment with SINGULAIR if rhinitis (SAR) in patients 2 years of age and older, and perennial such events occur (5.4 and 6.2). -

Montelukast and Neuropsychiatric Events in Children with Asthma: a Nested Case-Control Study
ORIGINAL ARTICLES Montelukast and Neuropsychiatric Events in Children with Asthma: A Nested Case–Control Study S. Dresden Glockler-Lauf, MPH1, Yaron Finkelstein, MD1,2,3,4,5, Jingqin Zhu, MSc1,2, Laura Y. Feldman, MPH1, and Teresa To, PhD1,2,6 Objective To examine the association between montelukast prescription and neuropsychiatric events in children with asthma. Study design A matched, nested case–control design was used to identify cases and controls from a cohort of children aged 5-18 years with physician-diagnosed asthma from 2004 to 2015, in Ontario, Canada, prescribed an asthma maintenance medication. Cases were children with a hospitalization or emergency department visit for a neuropsychiatric event. Cases were matched to up to 4 controls on birth year, year of asthma diagnosis, and sex. The exposures were dispensed prescriptions for montelukast (yes/no) and number of dispensed montelukast prescriptions in the year before the index date. Conditional logistic regression was used to measure the unadjusted OR and aOR and 95% CIs for montelukast prescription and neuropsychiatric events. Covariates in the adjusted model included sociodemographic factors and measures of asthma severity. Results In total, 898 cases with a neuropsychiatric event and 3497 matched controls were included. Children who experienced a new-onset neuropsychiatric event had nearly 2 times the odds of having been prescribed montelu- kast, compared with controls (OR 1.91, 95% CI 1.15-3.18; P = .01). Most cases presented for anxiety (48.6%) and/ or sleep disturbance (26.1%). Conclusions Children with asthma who experienced a new-onset neuropsychiatric event had nearly twice the odds of having been prescribed montelukast in the year before their event. -

Leukotriene Modifiers (Accolate®, Singulair®, Zileuton®)
Leukotriene Modifiers (Accolate®, Singulair®, Zileuton®) What are They? Leukotriene modifiers reduce swelling and inflammation in the airways to prevent asthma symptoms. You may not notice a change in your asthma symptoms for one to two week, after starting to use them. • These medicines will not stop a sudden asthma attack. Albuterol should be used for sudden asthma attacks. • Only regular daily use of the leukotriene modifiers will prevent asthma symptoms. • It is important that you do not stop or decrease the dose of these medications without contacting your doctor. How should they be used? • Zafirlukast (Accolate®) is a tablet that is taken by mouth twice a day on an empty stomach (1 hour before you eat or 2 hours after you eat). It is very important to take this medicine on an empty stomach. o Zafirlukast must be taken every day even if you don’t have asthma symptoms. If you forget to take your dose on time, do not take twice as much the next time. Take the regularly scheduled dose as soon as you remember, then get back to your regular schedule. o Side effects . Zafirlukast (Accolate®) causes very few side effects. Some people may have headaches, nausea, or diarrhea. Although these side effects are quite uncommon, it is important for you to let your doctor know if you are experiencing any of these while taking zafirlukast. • Montelukast (Singulair®) o A tablet that is taken once a day at nighttime. o You can take Montelukast with or without food. o Montelukast must be taken every day even if you don’t have asthma symptoms. -

Zileuton for the Prophylaxis and Chronic Treatment of Asthma
Asthma Zileuton for the Prophylaxis and Chronic Treatment of Asthma a report by Bradley E Chipps, MD, FAAP, FAAAAI, FCCP Capital Allergy and Respiratory Disease Center Zileuton is the only commercially available leukotriene synthesis inhibitor pulmonary function, and will be able to lead a normal life, carrying on all approved for the prophylaxis and treatment of chronic asthma in adults activities desired.1 Risk refers to the potential for negative outcomes, which and adolescents aged 12 years and above. According to the updated and include symptom exacerbations and associated urgent care (i.e. comprehensive 2007 Expert Panel Report 3 (EPR3): Guidelines for the unscheduled office visits, emergency department visits, hospitalizations), as Diagnosis and Management of Asthma, zileuton is an alternative well as any adverse events associated with treatment.1 therapeutic option to be added to inhaled corticosteroids (ICS) to enhance asthma control for patients who remain uncontrolled despite According to the EPR3, patients who have more than one exacerbation of using recommended therapy.1 This article will examine the role of symptoms a year requiring a short course of oral corticosteroids (OCS) are zileuton in asthma management in relation to asthma control as an considered not well controlled.1 Between May 2006 and May 2007 almost unmet patient need. 1.7 million asthma patients received at least one prescription for OCS. This represented the largest single reason for OCS use in the US.4 What should Uncontrolled Asthma Remains an Unmet Need the physician do when the patient’s asthma remains uncontrolled despite Despite almost 20 years of global and national guidelines for managing optimal therapy? The EPR3 presents a revised step-care approach to therapy, asthma, and despite scientific contributions to understanding its with six steps, each accounting for a progressive drop in asthma control.