COPD/Asthma Class Update
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
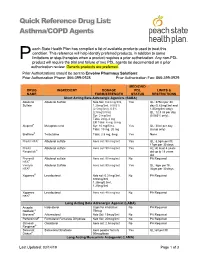
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Medicaid Policy Change
MEDICAID POLICY CHANGE IMMINENT PERIL JUSTIFICATION September 25, 2019 ADVAIR: POLICY CHANGE: LDH is changing the preferred drug list to switch the diskus inhaled powder from preferred to non-preferred and adding the HFA inhaler to the preferred list instead. JUSTIFICATION: This product is used to control symptoms and prevent complications caused by asthma or chronic obstructive pulmonary disease. This change is necessary to make an easier delivery device available for recipients to aid with treatment. Without preferred status, recipients would be required to obtain prior authorization which could delay necessary treatment. This change is needed by 10/1/19 due to the coming seasonal change in weather, including influenza and allergy season, that can significantly exacerbate chronic lung diseases, and so this presents an imminent peril to public health. EFFECTIVE DATE: October 1, 2019 LA Medicaid Preferred Drug List (PDL)/Non-Preferred Drug List (NPDL) Effective Date: July 15October 1, 2019 AG – Authorized Generic DR – Concurrent Prescriptions Must Be Written by Same Prescriber PU – Prior Use of Other Medication is Required AL – Age Limits DS – Maximum Days’ Supply Allowed QL – Quantity Limits BH – Behavioral Health Clinical Authorization Required for Children Younger Than 6 DT – Duration of Therapy Limit RX – Specific Prescription Requirements Years Old BY – Diagnosis Codes Bypass Some Requirements DX – Diagnosis Code Requirements TD – Therapeutic Duplication UN – Drug Use Not Warranted (Needs Appropriate CL – More Detailed Clinical Information -

Comparison of Intramuscular Betamethasone and Oral
Color profile: Disabled Composite Default screen 100 ORIGINAL ARTICLE 100 95 95 75 75 25 25 5 5 0 Comparison of intramuscular 0 betamethasone and oral prednisone in the prevention of relapse of acute asthma John S Chan MD1, Robert L Cowie MD1, Gerald C Lazarenko MD2, Cinde Little RRT1, Sandra Scott RRT1, Gordon T Ford MD FCCP1 1Division of Respiratory Medicine and 2Department of Emergency Medicine, University of Calgary, Calgary, Alberta JS Chan, RL Cowie, GC Lazarenko, C Little, S Scott, tively) and use of inhaled corticosteroids (46% versus 64.3% GT Ford. Comparison of intramuscular betamethasone respectively) (P<0.05). Using intention-to-treat analysis, the and oral prednisone in the prevention of relapse of acute relapse rates for betamethasone and prednisone at day 7 were asthma. Can Respir J 2001;8(3):147-152. 14.9% (13 of 87 patients) and 25% (21 of 84 patients), respectively (P=0.1), and at day 21, the rates were 36.8% (32 OBJECTIVE: To compare the relapse rate after a single intra- of 87 patients) and 31% (26 of 84 patients), respectively muscular injection of a long acting corticosteroid, betametha- (P=0.4). There were no differences in symptom score, peak sone, with oral prednisone in patients discharged from the flows and adverse effects between the two groups at days 7 emergency department (ED) for acute exacerbations of and 21. asthma. CONCLUSIONS: A single dose of intramuscular betametha- PATIENTS AND METHODS: Patients with acute exacer- sone 12 mg was safe and as efficacious as prednisone in pre- bations of asthma who were suitable for discharge from the venting the relapse of acute asthma. -

Long-Term Budesonide Or Nedocromil Mineral Accretion Over a Period of Years
term inhaled corticosteroid (ICS) treatment on bone Long-term Budesonide or Nedocromil mineral accretion over a period of years. Treatment, Once Discontinued, Does Not Alter the Course of Mild to Moderate Asthma in STUDY POPULATION. Cohort follow-up study for a median Children and Adolescents of 7 years with 877 children 5 to 12 years of age who Strunk RC, Sternberg AL, Szefler SJ, et al. J Pediatr. had mild-to-moderate asthma and initially were ran- 2009;154(5):682–687 domly assigned in the Childhood Asthma Management Program. PURPOSE OF THE STUDY. To determine whether long-term, METHODS. Serial dual-energy x-ray absorptiometry scans continuous use of inhaled antiinflammatory medications of the lumbar spine to assess bone mineral density were affects asthma outcomes in children with mild-to-mod- performed for all patients. Annual bone mineral accre- erate asthma after use is discontinued. tion was calculated for 531 boys and 346 girls. STUDY POPULATION. A total of 941 children, 5 to 12 years of RESULTS. Oral corticosteroid bursts produced dose- age, who had previously participated in the Childhood dependent reductions in bone mineral accretion (0.052, Asthma Management Program (CAMP). 0.049, and 0.046 g/cm2 per year with 0, 1–4, and Ն5 courses, respectively) and increases in the risk for osteo- METHODS. During the CAMP trial, subjects received treat- penia (10%, 14%, and 21%, respectively) in boys but ment with budesonide, nedocromil, or placebo for 4.3 not girls. Cumulative ICS use was associated with a small years. During the posttrial period, asthma manage- decrease in bone mineral accretion in boys but not girls ment was provided by primary care physicians ac- but no increased risk for osteopenia. -
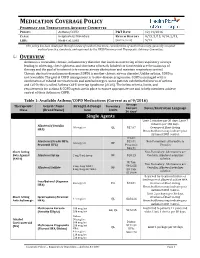
Medication Coverage Policy
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 12/14/2016 CLASS: Respiratory Disorders REVIEW HISTORY 9/15, 5/15, 9/14, 2/13, LOB: Medi-Cal, SJHA (MONTH/YEAR) 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee. OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 9/2016) Average Therapeutic Generic Name Strength & Dosage Formulary Cost per Limits Notes/Restriction Language Class (Brand Name) form 30 days* Single Agents Limit 2 inhalers per 30 days; Limit 7 -

Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma
Clinical Guideline for the Diagnosis, Evaluation and Management of Adults and Children with Asthma Color Key n Four Components of Asthma Care n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 0– 4 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children Aged 5–11 years n Classifying Asthma Severity, Assessing Asthma Control and the Stepwise Approach for Managing Asthma in Children >12 Years of Age & Adults n Long-Term Control Medications: Estimated Comparative Daily Dosages n Long-Term Control Medications: Usual Dosages n Quick-Relief Medications Guidelines are intended to be flexible. They serve as recommendations, not rigid criteria. Guidelines should be followed in most cases, but depending on the patient, and the circumstances, guidelines may need to be tailored to fit individual needs. 4750 11/18 Contents Criteria that suggest the diagnosis of asthma . 3 Goal of Therapy: Control of Asthma . 3 Four Components of Asthma Care 1. Assessment and Monitoring of Asthma Severity and Control . 4 2. Education for a Partnership in Care . 5 3. Control of Environmental Factors and Co-morbid Conditions that Affect Asthma . 5 4. Medications . 6 Bibliography . 6 Classifying Asthma Severity & Initiating Treatment in Children 0– 4 Years of Age . 7 Assessing Asthma Control & Adjusting Therapy in Children 0– 4 Years of Age . 7 Stepwise Approach for Managing Asthma in Children 0– 4 Years of Age . 8 Classifying Asthma Severity & Initiating Treatment in Children 5–11 Years of Age . 9 Assessing Asthma Control & Adjusting Therapy in Children 5–11 Years of Age . -

Us Anti-Doping Agency
2019U.S. ANTI-DOPING AGENCY WALLET CARDEXAMPLES OF PROHIBITED AND PERMITTED SUBSTANCES AND METHODS Effective Jan. 1 – Dec. 31, 2019 CATEGORIES OF SUBSTANCES PROHIBITED AT ALL TIMES (IN AND OUT-OF-COMPETITION) • Non-Approved Substances: investigational drugs and pharmaceuticals with no approval by a governmental regulatory health authority for human therapeutic use. • Anabolic Agents: androstenediol, androstenedione, bolasterone, boldenone, clenbuterol, danazol, desoxymethyltestosterone (madol), dehydrochlormethyltestosterone (DHCMT), Prasterone (dehydroepiandrosterone, DHEA , Intrarosa) and its prohormones, drostanolone, epitestosterone, methasterone, methyl-1-testosterone, methyltestosterone (Covaryx, EEMT, Est Estrogens-methyltest DS, Methitest), nandrolone, oxandrolone, prostanozol, Selective Androgen Receptor Modulators (enobosarm, (ostarine, MK-2866), andarine, LGD-4033, RAD-140). stanozolol, testosterone and its metabolites or isomers (Androgel), THG, tibolone, trenbolone, zeranol, zilpaterol, and similar substances. • Beta-2 Agonists: All selective and non-selective beta-2 agonists, including all optical isomers, are prohibited. Most inhaled beta-2 agonists are prohibited, including arformoterol (Brovana), fenoterol, higenamine (norcoclaurine, Tinospora crispa), indacaterol (Arcapta), levalbuterol (Xopenex), metaproternol (Alupent), orciprenaline, olodaterol (Striverdi), pirbuterol (Maxair), terbutaline (Brethaire), vilanterol (Breo). The only exceptions are albuterol, formoterol, and salmeterol by a metered-dose inhaler when used -

Difference Between Patient-Reported Side Effects of Ciclesonide Versus fluticasone Propionate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2010) 104, 1825e1833 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Difference between patient-reported side effects of ciclesonide versus fluticasone propionate Thys van der Molen a, Juliet M. Foster a,b, Manfred Caeser c,e, Thomas Mu¨ller c, Dirkje S. Postma d,* a Department of General Practice, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands b Woolcock Institute of Medical Research, 431 Glebe Point Rd, Glebe NSW 2037, Sydney, Australia c Nycomed GmbH, Byk-Gulden-Straße 2, 78467 Konstanz, Konstanz, Germany d Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands Received 18 January 2010; accepted 26 May 2010 Available online 2 July 2010 KEYWORDS Summary Adverse events; Rationale: Patient-reported outcomes provide new insights into the dynamics of asthma Inhaled corticosteroid management. Further to asthma control and quality of life, self-reported side effects of treat- questionnaire; ment can be assessed with the validated Inhaled Corticosteroid Questionnaire (ICQ). ICQ; Objectives: To compare patient-reported side effects between the inhaled corticosteroids Patient-reported ciclesonide and fluticasone propionate. outcomes Methods: Patients with moderate or moderate-to-severe asthma, pre-treated with a constant dose and type of medication, were randomized in three separate studies: 1) once daily cicle- sonide 320 mg(n Z 234) or twice daily fluticasone propionate 200 mg(n Z 240); 2) twice daily ciclesonide 320 mg(n Z 255) or twice daily fluticasone propionate 375 mg(n Z 273); and 3) twice daily ciclesonide 320 mg(n Z 259) or twice daily fluticasone propionate 500 mg (n Z 244). -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object.