Therapeutic Class Overview Intranasal Corticosteroids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Zhejiang Xianju Pharmaceutical Co. Ltd
No.1, Xianyao Road, Xianju, Zhejiang, China, 317300 Xianju Pharma Outline Outline I. Brief Introduction II. Quality Unit III. Production System IV. EHS System I. Brief Introduction Xianju Pharma Zhejiang Xianju Pharmaceutical Co., Ltd. A professional manufacturer of steroids and hormone products with largest scale and maximum varieties in China. A state-designated manufacturer of contraceptive drugs in China. Company Milestones Jan 1972 Foundation of company May 1997 Incorporated into Zhejiang Medicine Co., Ltd Oct. 1999 Listed in Shanghai Stock Market Jun. 2000 Reorganized into Xianju Pharmaceutical Co., Ltd Dec. 2001 Reformed to Zhejiang Xianju Pharmaceutical Co., Ltd Jan. 2010 listed in Shenzhen Stock Market Location of Xianju There are six airports around Shanghai Xianju, which makes us easily accessible for our partners. Headquarter Hangzhou Located in Xianju, Taizhou City Ningbo Yangfu Site (FPPs) Located in Yangfu, Xianju, Taizhou Yiwu City 6.8km from headquarter Duqiao Site (APIs) Located in LinHai, TaiZhou City, 82.9km from headquarter Taizhou Wenzhou Yangfu Site (APIs) Under construction, finish at 2017 Company Organization General Manager Vice G.M for Vice G.M Vice G.M for Vice G.M for Vice G.M for Quality Director Sales for Market Administration Finance Technology Finance Dept Finance Dept Application Tech Dept Endineering Construction Domestic DrugRegistrationDept. Research& Development Dept. Marketing Dept. Marketing Quality Control Quality Domestic Trading Dept International TradeDep Quality Assurance For FPP Quality Assurance For API Regulatory AffairsDept Human Resource Dept Information Technology Dept Dept Enterprise Management Dept Affairs Administrative Taizhou Xianju Quality System Quality Xianju Taizhou . t G.M. Assistant EHS Dept Production Management Dept G.M. -
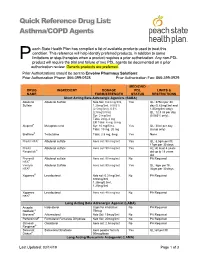
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

A Comparison of Fluticasone Propionate, 1 Mg Daily, with Beclomethasone Dipropionate, 2 Mg Daily, in the Treatment of Severe Asthma
Copyright ©ERS Joumals Ltd 1993 Eur Respir J , 1993, 6, Sn-884 European Respiratory Joumal Printed in UK - all rights reserved ISSN 0903 - 1936 A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma N.C. Bames*, G. Marone**, G.U. Di Maria***, S. Visser, I. Utama++, S.L. Payne+++, on behalf of an International Study Group A comparison of fluticasone propionate. 1 mg daily, with beclomethasone dipropionate, • The London Chest Hospital, London, 2 mg daily, in the treatment of severe asthma. N. C. Bames, G. Marone, G.U. Di Maria, UK. ** Servizio di Allergologia e S. Visser. l Utama, S.L Payne, on behalf of an International Study Group. @ERS Immunologia Clinica. I Clinica Medica Journals Ltd 1993. Universita, Napoli, Italy. *** lnstituto Malattie Respiratorie, Ospedale Tomaselli, ABSTRACT: We wanted w compare the efficacy and safety of fluticasone propi Catania, Sicily, Italy. onate, a new topically active inhaled corticosteroid, to that of high dose beclo + H.F. Verwoerd Hospital, Pretoria, South methasone dipropionate, in severe adult asthma. Africa. ++ St Laurentius Ziekenhius, 1 Patients currently receiving between 1.5-2.0 mg·day- of an inhaled corticoster CV Roermond, The Netherlands. +++ oid were treated for six weeks in a double-blind, randomized, parallel group study Glaxo Group Research Ltd, Greenford, with 1 mg·day-1 fluticasone propionate (n•82), or 2 mg·day·1 beclometbasone Middlesex, UK. dipropionate (n•72). Mean morning peak expirarory flow rates (PEFR) increased from 303 w 321 Correspondence: N.C. Bames l·min-1 with fluticasone propionate, and from 294 w 319 l·min·1 with beclometbasone The London Chest Hospital dipropionate. -

This Fact Sheet Provides Information to Patients with Eczema and Their Carers. About Topical Corticosteroids How to Apply Topic
This fact sheet provides information to patients with eczema and their carers. About topical corticosteroids You or your child’s doctor has prescribed a topical corticosteroid for the treatment of eczema. For treating eczema, corticosteroids are usually prepared in a cream or ointment and are applied topically (directly onto the skin). Topical corticosteroids work by reducing inflammation and helping to control an over-reactive response of the immune system at the site of eczema. They also tighten blood vessels, making less blood flow to the surface of the skin. Together, these effects help to manage the symptoms of eczema. There is a range of steroids that can be used to treat eczema, each with different strengths (potencies). On the next page, the potencies of some common steroids are shown, as well as the concentration that they are usually used in cream or ointment preparations. Using a moisturiser along with a steroid cream does not reduce the effect of the steroid. There are many misconceptions about the side effects of topical corticosteroids. However these treatments are very safe and patients are encouraged to follow the treatment regimen as advised by their doctor. How to apply topical corticosteroids How often should I apply? How much should I apply? Apply 1–2 times each day to the affected area Enough cream should be used so that the of skin according to your doctor’s instructions. entire affected area is covered. The cream can then be rubbed or massaged into the Once the steroid cream has been applied, inflamed skin. moisturisers can be used straight away if needed. -

Determination of Triamcinolone Acetonide Steroid on Glassy Carbon Electrode by Stripping Voltammetric Methods
Int. J. Electrochem. Sci., 3 (2008) 509-518 International Journal of ELECTROCHEM ICAL SCIENCE www.electrochemsci.org Determination of Triamcinolone Acetonide Steroid on Glassy Carbon Electrode by Stripping Voltammetric Methods C. Vedhi, R. Eswar, H. Gurumallesh Prabu and P. Manisankar* Department of Industrial Chemistry, Alagappa University, Karaikudi –630 003,Tamil Nadu, India *E-mail: [email protected] or [email protected] Received: 9 December 2007 / Accepted: 6 February 2008 / Online published: 20 February 2008 A corticosteroid, triamcinolone acetonide was determined by stripping voltammetric procedure using glassy carbon electrode. Cyclic voltammetric behaviour of steroid was studied in 50% aqueous methanol at acid, neutral and alkaline pH conditions. Influence of pH, sweep rate and steroid concentration were studied. An irreversible and adsorption-controlled well-defined reduction peak was observed in all pH conditions. The reduction peak potential shifted cathodically with change in pH. Controlled potential coulometry revealed two-electron reduction of the α,β-unsaturated carbonyl function present in the steroid. A systematic study of the experimental parameters that affect the differential pulse / square wave stripping voltammetric response was carried out and optimized conditions were arrived at. Under optimum conditions, differential pulse and square wave adsorptive stripping voltammetric procedures were developed for the determination of triamcinolone acetonide steroid at pH 13.0. A calibration plots was derived and the lower limit of determination observed are 0.1 µg/mL from DPSV and 0.01 µg/mL from SWSV. Keywords: Triamcinolone acetonide steroid, Cyclic Voltammetry, Stripping Voltammetry, Glassy carbon electrode. 1. INTRODUCTION Corticosteroids are hormones produced naturally by the adrenal glands, which have many important functions on every organ system. -

Antiviral Effect of Budesonide Against SARS-Cov-2
viruses Communication Antiviral Effect of Budesonide against SARS-CoV-2 Natalie Heinen 1 , Toni Luise Meister 1 , Mara Klöhn 1 , Eike Steinmann 1, Daniel Todt 1,2 and Stephanie Pfaender 1,* 1 Department of Molecular and Medical Virology, Ruhr-University Bochum, 44801 Bochum, Germany; [email protected] (N.H.); [email protected] (T.L.M.); [email protected] (M.K.); [email protected] (E.S.); [email protected] (D.T.) 2 European Virus Bioinformatics Center (EVBC), 07743 Jena, Germany * Correspondence: [email protected]; Tel.: +49-234-32-23189 Abstract: Treatment options for COVID-19, a disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, are currently severely limited. Therefore, antiviral drugs that efficiently reduce SARS-CoV-2 replication or alleviate COVID-19 symptoms are urgently needed. Inhaled glucocorticoids are currently being discussed in the context of treatment for COVID-19, partly based on a previous study that reported reduced recovery times in cases of mild COVID-19 after inhalative administration of the glucocorticoid budesonide. Given various reports that describe the potential antiviral activity of glucocorticoids against respiratory viruses, we aimed to analyze a potential antiviral activity of budesonide against SARS-CoV-2 and circulating variants of concern (VOC) B.1.1.7 (alpha) and B.1.351 (beta). We demonstrate a dose-dependent inhibition of SARS-CoV-2 that was comparable between all viral variants tested while cell viability remains unaffected. Our results are encouraging as they could indicate a multimodal mode of action of budesonide against SARS-CoV-2 and COVID-19, which could contribute to an improved clinical performance. -

Flonase Sensimist Allergy Relief (Fluticasone Furoate)
Flonase® Sensimist™ Allergy Relief (fluticasone furoate) – Rx-to-OTC Approval • On February 8, 2017, GlaxoSmithKline Consumer Healthcare announced the launch of Flonase Sensimist Allergy Relief (fluticasone furoate) nasal spray, as an over the counter (OTC) treatment to temporarily relieve symptoms of hay fever or other upper respiratory allergies: nasal congestion; runny nose; sneezing; itchy nose; and itchy, watery eyes (in ages 12 years and older). — Flonase Sensimist Allergy Relief contains 27.5 mcg/spray of fluticasone furoate. — Flonase Sensimist Allergy Relief should not be used in children less than 2 years of age. • Previously, fluticasone furoate was only available by prescription as Veramyst®. Veramyst is no longer commercially available. • Fluticasone is also available OTC as brand (Flonase® Allergy Relief, Children’s Flonase® Allergy Relief) and generic products containing 50 mcg/spray of fluticasone propionate. — These products share the same indications as Flonase Sensimist Allergy Relief, but are not intended for children under 4 years of age. • Prescription fluticasone propionate nasal spray (50 mcg/spray) is available generically and indicated for the management of the nasal symptoms of perennial non-allergic rhinitis in adult and pediatric patients aged 4 years and older. • Warnings for Flonase Sensimist Allergy Relief state the following: — Do not use: in children under 2 years of age, to treat asthma, if there is an injury or surgery to the nose that is not fully healed, or if an allergic reaction to Flonase Sensimist Allergy Relief or any of its ingredients has occurred. — Ask a doctor prior to use if the patient has or had glaucoma or cataracts. -

Superior Nuclear Receptor Selectivity and Therapeutic Index of Methylprednisolone Aceponate Versus Mometasone Furoate
DOI:10.1111/j.1600-0625.2007.00597.x www.blackwellpublishing.com/EXD Original Article Superior nuclear receptor selectivity and therapeutic index of methylprednisolone aceponate versus mometasone furoate Parham Mirshahpanah1, Wolf-Dietrich Do¨ cke2, Udo Merbold2, Khusru Asadullah2, Lars Ro¨se2, Heike Scha¨ cke2 and Thomas M. Zollner1 1Research Business Area Dermatology, Berlex Biosciences, Richmond, CA, USA; 2Corporate Research Area Inflammation, Bayer Schering Pharma, Berlin, Germany Correspondence: Thomas M. Zollner, TRG Inflammation, Bayer Schering Pharma, Berlin, Germany, Tel.: +1 510 669 4272, e-mail: [email protected] Accepted for publication 18 June 2007 Abstract: Although introduced more than 50 years ago, topical a relevant rodent model in vivo. We demonstrate that glucocorticoids are still the first line therapy for many methylprednisolone aceponate displays higher specificity in inflammatory skin disorders such as atopic eczema, contact nuclear receptor binding compared with mometasone furoate. dermatitis and many others. Recently, significant improvements Methylprednisolone aceponate was also markedly superior in have been made to optimize the ratio of desired to unwanted terms of minimizing induction of skin atrophy or telangiectasias effects. While with early compounds such as triamcinolone, when compared with mometasone furoate. Based on these topical side effects such as skin atrophy and telangiectasias can be observations, methylprednisolone aceponate is expected to have a observed rather frequently, newer drugs such as methyl- greater therapeutic index as compared with mometasone furoate, prednisolone aceponate or mometasone furoate have a at least in the test systems used here. The degree to which this significantly improved therapeutic index. The present study observation may translate into a clinical setting requires compared these two modern topical glucocorticoids, which confirmation. -

Difference Between Patient-Reported Side Effects of Ciclesonide Versus fluticasone Propionate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2010) 104, 1825e1833 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Difference between patient-reported side effects of ciclesonide versus fluticasone propionate Thys van der Molen a, Juliet M. Foster a,b, Manfred Caeser c,e, Thomas Mu¨ller c, Dirkje S. Postma d,* a Department of General Practice, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands b Woolcock Institute of Medical Research, 431 Glebe Point Rd, Glebe NSW 2037, Sydney, Australia c Nycomed GmbH, Byk-Gulden-Straße 2, 78467 Konstanz, Konstanz, Germany d Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands Received 18 January 2010; accepted 26 May 2010 Available online 2 July 2010 KEYWORDS Summary Adverse events; Rationale: Patient-reported outcomes provide new insights into the dynamics of asthma Inhaled corticosteroid management. Further to asthma control and quality of life, self-reported side effects of treat- questionnaire; ment can be assessed with the validated Inhaled Corticosteroid Questionnaire (ICQ). ICQ; Objectives: To compare patient-reported side effects between the inhaled corticosteroids Patient-reported ciclesonide and fluticasone propionate. outcomes Methods: Patients with moderate or moderate-to-severe asthma, pre-treated with a constant dose and type of medication, were randomized in three separate studies: 1) once daily cicle- sonide 320 mg(n Z 234) or twice daily fluticasone propionate 200 mg(n Z 240); 2) twice daily ciclesonide 320 mg(n Z 255) or twice daily fluticasone propionate 375 mg(n Z 273); and 3) twice daily ciclesonide 320 mg(n Z 259) or twice daily fluticasone propionate 500 mg (n Z 244). -

Canine Vascular Tissues Are Targets for Androgens, Estrogens, Progestins, and Glucocorticoids
Canine vascular tissues are targets for androgens, estrogens, progestins, and glucocorticoids. K B Horwitz, L D Horwitz J Clin Invest. 1982;69(4):750-758. https://doi.org/10.1172/JCI110513. Research Article Sex differences and steroid hormones are known to influence the vascular system as shown by the different incidence of atherosclerosis in men and premenopausal women, or by the increased risk of cardiovascular diseases in women taking birth control pills or men taking estrogens. However, the mechanisms for these effects in vascular tissues are not known. Since steroid actions in target tissues are mediated by receptors, we have looked for cytoplasmic steroid receptor proteins in vascular tissues of dogs. We find specific saturable receptors, sedimenting at 8S on sucrose density gradients for estrogens (measured with [3H]estradiol +/- unlabeled diethylstilbestrol), androgens (measured with [3H]R1881 +/- unlabeled R1881 and triamcinolone acetonide), and glucocorticoids (measured with [3H]dexamethasone +/- unlabeled dexamethasone); they are absent for progesterone (measured with [3H]R5020 +/- unlabeled R5020 and dihydrotestosterone). Progesterone receptors can, however, be induced by 1-wk treatment of dogs with physiological estradiol concentrations (100 pg/ml serum estrogen), indicating a functional estrogen receptor. Receptor levels range from 20 to 2,000 fmol/mg DNA. They are specific for each hormone; unrelated steroids fail to complete for binding. Low dissociation constants, measured by Scatchard analyses, show that binding is of high affinity. Steroid binding sites are in the media and/or adventitia since they persist when the intima is removed. Compared with the arteries, receptor levels are reduced 80% in inferior venae cavae of […] Find the latest version: https://jci.me/110513/pdf Canine Vascular Tissues Are Targets for Androgens, Estrogens, Progestins, and Glucocorticoids KATHRYN B. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TRELEGY ELLIPTA 100/62.5/25 fluticasone furoate (100 micrograms)/umeclidinium (as bromide) (62.5 micrograms)/vilanterol (as trifenatate) (25 micrograms), powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 92 micrograms fluticasone furoate, 55 micrograms umeclidinium (equivalent to 65 micrograms umeclidinium [as bromide]) and 22 micrograms vilanterol (as trifenatate). This corresponds to a pre-dispensed dose of 100 micrograms fluticasone furoate, 62.5 micrograms umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide) and 25 micrograms vilanterol (as trifenatate). Excipient with known effect: Each delivered dose contains approximately 25 milligrams of lactose (as monohydrate). For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for inhalation. White powder in a light grey inhaler (Ellipta) with a beige mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications TRELEGY ELLIPTA is indicated for the maintenance treatment of adults with moderate to severe chronic obstructive pulmonary disease (COPD) who require treatment with a long- acting muscarinic receptor antagonist (LAMA) + long-acting beta2-receptor agonist (LABA) + inhaled corticosteroid (ICS). TRELEGY ELLIPTA should not be used for the initiation of COPD treatment. 4.2. Dose and method of administration Patients can be changed from their existing inhalers to TRELEGY ELLIPTA at the next dose. However it is important that patients do not take other LABA or LAMA or ICS while taking TRELEGY ELLIPTA. A stepwise approach to the management of COPD is recommended, including the cessation of smoking and a pulmonary rehabilitation program. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid.