Pharmacy Medical Necessity Guidelines: Nasal Corticosteroid Medications Effective: May 11, 2021
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
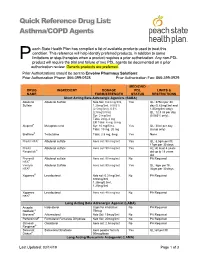
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

Flonase Sensimist Allergy Relief (Fluticasone Furoate)
Flonase® Sensimist™ Allergy Relief (fluticasone furoate) – Rx-to-OTC Approval • On February 8, 2017, GlaxoSmithKline Consumer Healthcare announced the launch of Flonase Sensimist Allergy Relief (fluticasone furoate) nasal spray, as an over the counter (OTC) treatment to temporarily relieve symptoms of hay fever or other upper respiratory allergies: nasal congestion; runny nose; sneezing; itchy nose; and itchy, watery eyes (in ages 12 years and older). — Flonase Sensimist Allergy Relief contains 27.5 mcg/spray of fluticasone furoate. — Flonase Sensimist Allergy Relief should not be used in children less than 2 years of age. • Previously, fluticasone furoate was only available by prescription as Veramyst®. Veramyst is no longer commercially available. • Fluticasone is also available OTC as brand (Flonase® Allergy Relief, Children’s Flonase® Allergy Relief) and generic products containing 50 mcg/spray of fluticasone propionate. — These products share the same indications as Flonase Sensimist Allergy Relief, but are not intended for children under 4 years of age. • Prescription fluticasone propionate nasal spray (50 mcg/spray) is available generically and indicated for the management of the nasal symptoms of perennial non-allergic rhinitis in adult and pediatric patients aged 4 years and older. • Warnings for Flonase Sensimist Allergy Relief state the following: — Do not use: in children under 2 years of age, to treat asthma, if there is an injury or surgery to the nose that is not fully healed, or if an allergic reaction to Flonase Sensimist Allergy Relief or any of its ingredients has occurred. — Ask a doctor prior to use if the patient has or had glaucoma or cataracts. -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TRELEGY ELLIPTA 100/62.5/25 fluticasone furoate (100 micrograms)/umeclidinium (as bromide) (62.5 micrograms)/vilanterol (as trifenatate) (25 micrograms), powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 92 micrograms fluticasone furoate, 55 micrograms umeclidinium (equivalent to 65 micrograms umeclidinium [as bromide]) and 22 micrograms vilanterol (as trifenatate). This corresponds to a pre-dispensed dose of 100 micrograms fluticasone furoate, 62.5 micrograms umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide) and 25 micrograms vilanterol (as trifenatate). Excipient with known effect: Each delivered dose contains approximately 25 milligrams of lactose (as monohydrate). For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for inhalation. White powder in a light grey inhaler (Ellipta) with a beige mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications TRELEGY ELLIPTA is indicated for the maintenance treatment of adults with moderate to severe chronic obstructive pulmonary disease (COPD) who require treatment with a long- acting muscarinic receptor antagonist (LAMA) + long-acting beta2-receptor agonist (LABA) + inhaled corticosteroid (ICS). TRELEGY ELLIPTA should not be used for the initiation of COPD treatment. 4.2. Dose and method of administration Patients can be changed from their existing inhalers to TRELEGY ELLIPTA at the next dose. However it is important that patients do not take other LABA or LAMA or ICS while taking TRELEGY ELLIPTA. A stepwise approach to the management of COPD is recommended, including the cessation of smoking and a pulmonary rehabilitation program. -

Asthma Medications
Relievers / Rescue / Bronchodilators Asthma Short-acting Beta Agonists 2 Medications Ipratropium Bromide ProAir ProAir RespiClick Proventil Ventolin Xopenex albuterol sulfate albuterol sulfate dry powder albuterol sulfate albuterol sulfate levalbuterol tartrate 90mcg 90mcg 90mcg 90mcg 45mcg Teva Teva Merck GlaxoSmithKline Sunovion Atrovent* Combivent Respimat* ipratropium bromide ipratropium bromide 20mcg, 17mcg albuterol sulfate 100mcg Boehringer Ingelheim Boehringer Ingelheim Nebulized Albuterol Xopenex Inhalation Solution Xopenex Inhalation Solution Xopenex Inhalation Solution * Ipratropium bromide is not a recommended rescue inhaler albuterol sulfate levalbuterol HCl levalbuterol HCl levalbuterol HCl outside of use in the emergency room or urgent care but may, 2.5mg/3mL 0.31mg/3mL 0.63mg/3mL 1.25mg/3mL on occasion, be prescribed to supplement short-acting Beta 2 generic Sunovion Sunovion Sunovion agonists. Controllers Inhaled Corticosteroids (ICS): Metered-Dose Inhalers (MDI) Aerospan Alvesco Alvesco Asmanex Asmanex Flovent Flovent Flovent flunisolide ciclesonide ciclesonide mometasone furoate mometasone furoate fluticasone propionate fluticasone fluticasone propionate 80mcg 80mcg 160mcg 100mcg 200mcg 44mcg propionate 220mcg Meda Pharmaceuticals Sunovion Sunovion Merck Merck GlaxoSmithKline 110mcg GlaxoSmithKline GlaxoSmithKline Inhaled Corticosteroids (ICS): Dry Powder Inhalers(continued on back) QVAR QVAR beclomethasone beclomethasone dipropionate dipropionate 40mcg 80mcg ArmonAir RespiClick ArmonAir RespiClick ArmonAir RespiClick -

Utah Medicaid Pharmacy and Therapeutics Committee Drug
Utah Medicaid Pharmacy and Therapeutics Committee Drug Class Review Single Ingredient Nasal Corticosteroids Beclomethasone dipropionate (Qnasl) Beclomethasone dipropionate monohydrate (Beconase AQ) Budesonide (Rhinocort) Ciclesonide (Omnaris, Zetonna) Flunisolide (Generic) Fluticasone Furoate (Flonase Sensimist) Fluticasone Propionate (Flonase, Xhance) Mometasone Furoate (Nasonex) Triamcinolone Acetonide (Nasacort) AHFS Classification: 52.08.08 Corticosteroids (EENT) Final Report February 2018 Review prepared by: Valerie Gonzales, Pharm.D., Clinical Pharmacist Elena Martinez Alonso, B.Pharm., MSc MTSI, Medical Writer Vicki Frydrych, Pharm.D., Clinical Pharmacist Joanita Lake, B.Pharm., MSc EBHC (Oxon), Research Assistant Professor Joanne LaFleur, Pharm.D., MSPH, Associate Professor University of Utah College of Pharmacy University of Utah College of Pharmacy, Drug Regimen Review Center Copyright © 2018 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved Contents Abbreviations ................................................................................................................................................ 2 Executive Summary ....................................................................................................................................... 3 Introduction .................................................................................................................................................. 5 Table 1. Nasal corticosteroid products ............................................................................................. -

Drug Class Review Nasal Corticosteroids
Drug Class Review Nasal Corticosteroids Final Report Update 1 June 2008 The Agency for Healthcare Research and Quality has not yet seen or approved this report The purpose of this report is to make available information regarding the comparative effectiveness and safety profiles of different drugs within pharmaceutical classes. Reports are not usage guidelines, nor should they be read as an endorsement of, or recommendation for, any particular drug, use or approach. Oregon Health & Science University does not recommend or endorse any guideline or recommendation developed by users of these reports. Dana Selover, MD Tracy Dana, MLS Colleen Smith, PharmD Kim Peterson, MS Oregon Evidence-based Practice Center Oregon Health & Science University Mark Helfand, MD, MPH, Director Marian McDonagh, PharmD, Principal Investigator, Drug Effectiveness Review Project Copyright © 2008 by Oregon Health & Science University Portland, Oregon 97239. All rights reserved. Final Report Update 1 Drug Effectiveness Review Project TABLE OF CONTENTS INTRODUCTION ..........................................................................................................................5 Scope and Key Questions .......................................................................................................................7 METHODS ....................................................................................................................................9 Literature Search .....................................................................................................................................9 -

Formulary (List of Covered Drugs)
2018 Formulary (List of Covered Drugs) PLEASE READ: THIS DOCUMENT CONTAINS INFORMATION ABOUT THE DRUGS WE COVER IN THIS PLAN Assurance Rx (HMO-POS) Essence Rx (HMO-POS) Esteem Rx (HMO-POS) Promise Rx (HMO-POS) Spirit Rx (HMO-POS) Surety Rx (HMO-POS) This formulary was updated on Nov. 21, 2018. For more recent information or other questions, please contact Security Health Plan Customer Service at 1-877-998-0998 or, for TTY users, 711, or visit https://www.securityhealth.org/medicareformulary. We are open 7 days a week, 8 a.m. to 8 p.m., from Oct. 1-March 31; and Monday through Friday, 8 a.m. to 8 p.m., from April 1-Sept. 30. Y0117_MC-778-0628-C-09-18 Effective date 12/1/2018 Updated 11/21/2018 Formulary ID 00018450, v.20 Note to existing members: This formulary has changed since last year. Please review this document to make sure that it still contains the drugs you take. When this drug list (formulary) refers to “we,” “us,” or “our,” it means Security Health Plan. When it refers to “plan” or “our plan,” it means Assurance Rx (HMO-POS), Essence Rx (HMO-POS), Promise Rx (HMO-POS), Spirit Rx (HMO- POS) or Surety Rx (HMO-POS). This document includes a list of the drugs (formulary) for our plan which is current as of Dec. 1, 2018. For an updated formulary, please contact us. Our contact information, along with the date we last updated the formulary, appears on the front and back cover pages. You must generally use network pharmacies to use your prescription drug benefit. -

Steroids: the Good, the Bad and the Ugly!
STEROIDS: THE GOOD, THE BAD AND THE UGLY! MICHAEL ZACHARISEN, MD. CONFLICT OF INTEREST, DISCLOSURES Conflict of interest: None Disclosures: I prescribe steroids and have for 31 years I will be discussing off label uses of steroids 1997 the first ICS OUTLINE • History of steroids in asthma • Steroids: Types, preparations, routes of administration • “The Good”: Benefits of steroids in asthma • “The Bad and the Ugly”: Risks and side effects of steroids in asthma • How to mitigate the risks of steroids when used in asthma DISEASES TREATED WITH CORTICOSTEROIDS Inflammatory • Asthma Autoimmune Connective Tissue • Anaphylaxis • Systemic Lupus erythematosus (Lupus) • Hypersensitivity pneumonitis • Sarcoid, Systemic sclerosis, MCTD • ABPA • Inflammatory Bowel Disease • Urticaria (hives) • Vasculitis, Myositis • Eczema • Bullous dermatitis Immune Suppression Other • Cancer • Adrenal insufficiency/Addison’s CONTRAINDICATIONS FOR SYSTEMIC STEROIDS Absolute Relative • Systemic fungal infection • Hypertension and Congestive Heart Failure • Herpes simplex keratitis • Psychosis or depression • Hypersensitivity • Active peptic ulcer disease • Active TB • Diabetes mellitus • Osteoporosis • Cataracts, glaucoma • Recent intestinal anastomoses HOW STEROIDS WORK At the cell level: • Suppress multiple inflammatory genes that are activated in asthmatic airways by reversing histone acetylation of the activated inflammatory genes • Induce apoptosis of eosinophils • Upregulate beta-receptors HISTORY OF STEROIDS FOR ASTHMA/ALLERGY • 1900: Cortisone discovered (not -

Fluticasone Fuorate GSK, INN-Fluticasone Furoate
ANNEX I SUMMARY OF PRODUCT CHARACTERISTICS 1 1. NAME OF THE MEDICINAL PRODUCT FLUTICASONE FUROATE GSK 27.5 micrograms/spray nasal spray suspension 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each spray actuation delivers 27.5 micrograms of fluticasone furoate. For a full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Nasal spray, suspension. White suspension. 4. CLINICAL PARTICULARS 4.1 Therapeutic indications Adults, adolescents (12 years and over) and children (6 – 11 years) Fluticasone furoate GSK is indicated for the treatment of: • the symptoms of allergic rhinitis 4.2 Posology and method of administration Fluticasone furoate nasal spray is for administration by the intranasal route only. For full therapeutic benefit regular, scheduled usage is recommended. Onset of action has been observed as early as 8 hours after initial administration. However, it may take several days of treatment to achieve maximum benefit, and the patient should be informed that their symptoms will improve with continuous regular use (see section 5.1). The duration of treatment should be restricted to the period that corresponds to allergenic exposure. Adults and Adolescents (12 years and over) The recommended starting dose is two spray actuations (27.5 micrograms of fluticasone furoate per spray actuation) in each nostril once daily (total daily dose, 110 micrograms). Once adequate control of symptoms is achieved, dose reduction to one spray actuation in each nostril (total daily dose 55 micrograms) may be effective for maintenance. Children (6 to 11 years of age) The recommended starting dose is one spray actuation (27.5 micrograms of fluticasone furoate per spray actuation) in each nostril once daily (total daily dose, 55 micrograms). -

TITLE: Fluticasone Furoate Versus Fluticasone Propionate for Seasonal Allergic Rhinitis: a Review of the Clinical and Cost-Effectiveness
TITLE: Fluticasone Furoate versus Fluticasone Propionate for Seasonal Allergic Rhinitis: A Review of the Clinical and Cost-Effectiveness DATE: 13 June 2011 CONTEXT AND POLICY ISSUES: Seasonal allergic rhinitis is a common disorder with an US prevalence of about 10-30% in adults and 40% in children, affecting close to 60 million people.1,2 The Canadian Allergy, Asthma and Immunology Foundation estimates that 20-25% of Canadians have allergic rhinitis.3 In addition to allergen avoidance and immunotherapy, antihistamines and corticosteroids nasal spray are used to control nasal and ocular symptoms.4,5 Fluticasone furoate nasal spray (Avamys™ by GlaxoSmythKline Inc.) was approved by Health Canada in August 2007 for the treatment of seasonal allergic rhinitis.6 The efficacy of fluticasone furoate nasal spray for the treatment of nasal and ocular symptoms of allergic rhinitis was shown in a recent systematic review,7 as well as in randomized, double-blind, placebo- controlled studies.8,9 Fluticasone furoate nasal spray was not associated with hypothalamic- pituitary-adrenal axis suppression as shown in a randomized, double blind, placebo- and active- controlled (prednisone) study.10 To help in the consideration of formulary coverage of fluticasone furoate (Avamys™) for seasonal allergic rhinitis, this report compares the clinical and cost-effectiveness of fluticasone furoate with fluticasone propionate for the treatment of seasonal allergic rhinitis. RESEARCH QUESTIONS: 1) What is the clinical effectiveness of fluticasone furoate for seasonal allergic rhinitis as compared to fluticasone propionate? 2) What is the cost-effectiveness of fluticasone furoate for seasonal allergic rhinitis as compared to fluticasone propionate? Disclaimer: The Rapid Response Service is an information service for those involved in planning and providing health care in Canada. -

Aerospan (Flunisolide HFA, 80 Mcg) Inhalation Aerosol Use with AEROSPAN Canister Only for Oral Inhalation Only
AEROSPANTM (flunisolide HFA, 80 mcg) Inhalation Aerosol For Oral Inhalation Only Rx Only DESCRIPTION Flunisolide hemihydrate, the active component of AEROSPANTM (flunisolide HFA, 80 mcg) Inhalation Aerosol, is a corticosteroid having the chemical name 6α-Fluoro-11β, 16α, 17, 21 –tetrahydroxylpregna-1, 4-diene-3, 20-dione cyclic-16, 17-acetal with acetone, hemihydrate and the following chemical structure: O CH3 HO O C CH3 HO O . 1/2 H2O O F Flunisolide Hemihydrate Flunisolide hemihydrate is a white to creamy white crystalline powder with a molecular weight of 443.51 and an empirical formula of C24H31O6F •½ H2O. It is soluble in acetone, ethyl alcohol and HFA-134a and practically insoluble in water. AEROSPAN Inhalation Aerosol is a pressurized, metered-dose inhaler unit intended for oral inhalation only. The inhaler unit consists of a metal canister, a purple actuator, and a gray spacer. Each unit contains a 0.24 % w/w solution of flunisolide hemihydrate in 10:90 w/w ethanol:1,1,1,2-tetrafluoroethane (HFA 134a). After priming, each actuation delivers 139 mcg of flunisolide hemihydrate in 58 mg of solution from the canister valve and 80 mcg of flunisolide hemihydrate (equivalent to 78 mcg flunisolide) from the spacer at a flow rate of 30 L/min for 4 seconds. Using an in-vitro method at a fixed volume of 2 L, each actuation at the beginning of canister content delivers from the spacer 76 mcg (95% of the label claim) at a flow rate of 30 L/min, 61 mcg (76% of the label claim) at a flow rate of 20 L/min, 85 mcg (106% of the label claim) at a flow rate of 40 L/min, and 96 mcg (120% of the label claim) at a flow rate of 60 L/min. -
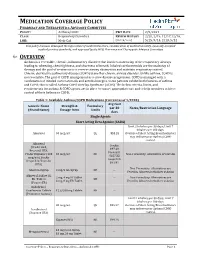
Asthma COPD and Asthma-COPD Overlap Syndrome (ACOS)
MEDICATION COVERAGE POLICY PHARMACY AND THERAPEUTICS ADVISORY COMMITTEE POLICY: Asthma/COPD P&T DATE 2/9/2021 CLASS: Respiratory Disorders REVIEW HISTORY 2/20, 2/19, 12/17,12/16, LOB: Medi-Cal (MONTH/YEAR) 5/15, 9/14, 2/13, 5/12 This policy has been developed through review of medical literature, consideration of medical necessity, generally accepted medical practice standards, and approved by the HPSJ Pharmacy and Therapeutic Advisory Committee OVERVIEW Asthma is a reversible, chronic, inflammatory disorder that involves narrowing of the respiratory airways leading to wheezing, chest tightness, and shortness of breath. Inhaled corticosteroids are the mainstay of therapy and the goal of treatment is to reverse airway obstruction and maintain respiratory control. Chronic obstructive pulmonary disease (COPD) is another chronic airway disorder. Unlike asthma, COPD is not reversible. The goal of COPD management is to slow disease progression. COPD is managed with a combination of inhaled corticosteroids and anticholinergics. Some patients exhibit both features of asthma and COPD; this is called Asthma-COPD Overlap Syndrome (ACOS). The below criteria, limits, and requirements for asthma & COPD agents are in place to ensure appropriate use and to help members achieve control of their Asthma or COPD. Table 1: Available Asthma/COPD Medications (Current as of 1/2020) Avg Cost Generic Name Strength & Formulary per 30 Notes/Restriction Language (Brand Name) Dosage form Limits days Single Agents Short Acting Beta Agonist (SABA) Limit 2 inhalers per 30 days; Limit 7 inhalers per 180 days. Albuterol 90 mcg/act QL $53.28 Overuse of Short Acting Bronchodilators may indicate poor Asthma/COPD control.