Therapeutic Class Overview Intranasal Corticosteroids
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-
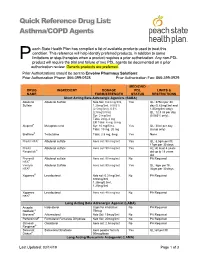
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

A Comparison of Fluticasone Propionate, 1 Mg Daily, with Beclomethasone Dipropionate, 2 Mg Daily, in the Treatment of Severe Asthma
Copyright ©ERS Joumals Ltd 1993 Eur Respir J , 1993, 6, Sn-884 European Respiratory Joumal Printed in UK - all rights reserved ISSN 0903 - 1936 A comparison of fluticasone propionate, 1 mg daily, with beclomethasone dipropionate, 2 mg daily, in the treatment of severe asthma N.C. Bames*, G. Marone**, G.U. Di Maria***, S. Visser, I. Utama++, S.L. Payne+++, on behalf of an International Study Group A comparison of fluticasone propionate. 1 mg daily, with beclomethasone dipropionate, • The London Chest Hospital, London, 2 mg daily, in the treatment of severe asthma. N. C. Bames, G. Marone, G.U. Di Maria, UK. ** Servizio di Allergologia e S. Visser. l Utama, S.L Payne, on behalf of an International Study Group. @ERS Immunologia Clinica. I Clinica Medica Journals Ltd 1993. Universita, Napoli, Italy. *** lnstituto Malattie Respiratorie, Ospedale Tomaselli, ABSTRACT: We wanted w compare the efficacy and safety of fluticasone propi Catania, Sicily, Italy. onate, a new topically active inhaled corticosteroid, to that of high dose beclo + H.F. Verwoerd Hospital, Pretoria, South methasone dipropionate, in severe adult asthma. Africa. ++ St Laurentius Ziekenhius, 1 Patients currently receiving between 1.5-2.0 mg·day- of an inhaled corticoster CV Roermond, The Netherlands. +++ oid were treated for six weeks in a double-blind, randomized, parallel group study Glaxo Group Research Ltd, Greenford, with 1 mg·day-1 fluticasone propionate (n•82), or 2 mg·day·1 beclometbasone Middlesex, UK. dipropionate (n•72). Mean morning peak expirarory flow rates (PEFR) increased from 303 w 321 Correspondence: N.C. Bames l·min-1 with fluticasone propionate, and from 294 w 319 l·min·1 with beclometbasone The London Chest Hospital dipropionate. -

Antiviral Effect of Budesonide Against SARS-Cov-2
viruses Communication Antiviral Effect of Budesonide against SARS-CoV-2 Natalie Heinen 1 , Toni Luise Meister 1 , Mara Klöhn 1 , Eike Steinmann 1, Daniel Todt 1,2 and Stephanie Pfaender 1,* 1 Department of Molecular and Medical Virology, Ruhr-University Bochum, 44801 Bochum, Germany; [email protected] (N.H.); [email protected] (T.L.M.); [email protected] (M.K.); [email protected] (E.S.); [email protected] (D.T.) 2 European Virus Bioinformatics Center (EVBC), 07743 Jena, Germany * Correspondence: [email protected]; Tel.: +49-234-32-23189 Abstract: Treatment options for COVID-19, a disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection, are currently severely limited. Therefore, antiviral drugs that efficiently reduce SARS-CoV-2 replication or alleviate COVID-19 symptoms are urgently needed. Inhaled glucocorticoids are currently being discussed in the context of treatment for COVID-19, partly based on a previous study that reported reduced recovery times in cases of mild COVID-19 after inhalative administration of the glucocorticoid budesonide. Given various reports that describe the potential antiviral activity of glucocorticoids against respiratory viruses, we aimed to analyze a potential antiviral activity of budesonide against SARS-CoV-2 and circulating variants of concern (VOC) B.1.1.7 (alpha) and B.1.351 (beta). We demonstrate a dose-dependent inhibition of SARS-CoV-2 that was comparable between all viral variants tested while cell viability remains unaffected. Our results are encouraging as they could indicate a multimodal mode of action of budesonide against SARS-CoV-2 and COVID-19, which could contribute to an improved clinical performance. -

Flonase Sensimist Allergy Relief (Fluticasone Furoate)
Flonase® Sensimist™ Allergy Relief (fluticasone furoate) – Rx-to-OTC Approval • On February 8, 2017, GlaxoSmithKline Consumer Healthcare announced the launch of Flonase Sensimist Allergy Relief (fluticasone furoate) nasal spray, as an over the counter (OTC) treatment to temporarily relieve symptoms of hay fever or other upper respiratory allergies: nasal congestion; runny nose; sneezing; itchy nose; and itchy, watery eyes (in ages 12 years and older). — Flonase Sensimist Allergy Relief contains 27.5 mcg/spray of fluticasone furoate. — Flonase Sensimist Allergy Relief should not be used in children less than 2 years of age. • Previously, fluticasone furoate was only available by prescription as Veramyst®. Veramyst is no longer commercially available. • Fluticasone is also available OTC as brand (Flonase® Allergy Relief, Children’s Flonase® Allergy Relief) and generic products containing 50 mcg/spray of fluticasone propionate. — These products share the same indications as Flonase Sensimist Allergy Relief, but are not intended for children under 4 years of age. • Prescription fluticasone propionate nasal spray (50 mcg/spray) is available generically and indicated for the management of the nasal symptoms of perennial non-allergic rhinitis in adult and pediatric patients aged 4 years and older. • Warnings for Flonase Sensimist Allergy Relief state the following: — Do not use: in children under 2 years of age, to treat asthma, if there is an injury or surgery to the nose that is not fully healed, or if an allergic reaction to Flonase Sensimist Allergy Relief or any of its ingredients has occurred. — Ask a doctor prior to use if the patient has or had glaucoma or cataracts. -

Difference Between Patient-Reported Side Effects of Ciclesonide Versus fluticasone Propionate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2010) 104, 1825e1833 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Difference between patient-reported side effects of ciclesonide versus fluticasone propionate Thys van der Molen a, Juliet M. Foster a,b, Manfred Caeser c,e, Thomas Mu¨ller c, Dirkje S. Postma d,* a Department of General Practice, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands b Woolcock Institute of Medical Research, 431 Glebe Point Rd, Glebe NSW 2037, Sydney, Australia c Nycomed GmbH, Byk-Gulden-Straße 2, 78467 Konstanz, Konstanz, Germany d Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands Received 18 January 2010; accepted 26 May 2010 Available online 2 July 2010 KEYWORDS Summary Adverse events; Rationale: Patient-reported outcomes provide new insights into the dynamics of asthma Inhaled corticosteroid management. Further to asthma control and quality of life, self-reported side effects of treat- questionnaire; ment can be assessed with the validated Inhaled Corticosteroid Questionnaire (ICQ). ICQ; Objectives: To compare patient-reported side effects between the inhaled corticosteroids Patient-reported ciclesonide and fluticasone propionate. outcomes Methods: Patients with moderate or moderate-to-severe asthma, pre-treated with a constant dose and type of medication, were randomized in three separate studies: 1) once daily cicle- sonide 320 mg(n Z 234) or twice daily fluticasone propionate 200 mg(n Z 240); 2) twice daily ciclesonide 320 mg(n Z 255) or twice daily fluticasone propionate 375 mg(n Z 273); and 3) twice daily ciclesonide 320 mg(n Z 259) or twice daily fluticasone propionate 500 mg (n Z 244). -

New Zealand Data Sheet
NEW ZEALAND DATA SHEET 1. PRODUCT NAME TRELEGY ELLIPTA 100/62.5/25 fluticasone furoate (100 micrograms)/umeclidinium (as bromide) (62.5 micrograms)/vilanterol (as trifenatate) (25 micrograms), powder for inhalation 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each delivered dose (the dose leaving the mouthpiece of the inhaler) contains 92 micrograms fluticasone furoate, 55 micrograms umeclidinium (equivalent to 65 micrograms umeclidinium [as bromide]) and 22 micrograms vilanterol (as trifenatate). This corresponds to a pre-dispensed dose of 100 micrograms fluticasone furoate, 62.5 micrograms umeclidinium (equivalent to 74.2 micrograms umeclidinium bromide) and 25 micrograms vilanterol (as trifenatate). Excipient with known effect: Each delivered dose contains approximately 25 milligrams of lactose (as monohydrate). For the full list of excipients, see Section 6.1 List of excipients. 3. PHARMACEUTICAL FORM Powder for inhalation. White powder in a light grey inhaler (Ellipta) with a beige mouthpiece cover and a dose counter. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications TRELEGY ELLIPTA is indicated for the maintenance treatment of adults with moderate to severe chronic obstructive pulmonary disease (COPD) who require treatment with a long- acting muscarinic receptor antagonist (LAMA) + long-acting beta2-receptor agonist (LABA) + inhaled corticosteroid (ICS). TRELEGY ELLIPTA should not be used for the initiation of COPD treatment. 4.2. Dose and method of administration Patients can be changed from their existing inhalers to TRELEGY ELLIPTA at the next dose. However it is important that patients do not take other LABA or LAMA or ICS while taking TRELEGY ELLIPTA. A stepwise approach to the management of COPD is recommended, including the cessation of smoking and a pulmonary rehabilitation program. -

Steroid Use in Prednisone Allergy Abby Shuck, Pharmd Candidate
Steroid Use in Prednisone Allergy Abby Shuck, PharmD candidate 2015 University of Findlay If a patient has an allergy to prednisone and methylprednisolone, what (if any) other corticosteroid can the patient use to avoid an allergic reaction? Corticosteroids very rarely cause allergic reactions in patients that receive them. Since corticosteroids are typically used to treat severe allergic reactions and anaphylaxis, it seems unlikely that these drugs could actually induce an allergic reaction of their own. However, between 0.5-5% of people have reported any sort of reaction to a corticosteroid that they have received.1 Corticosteroids can cause anything from minor skin irritations to full blown anaphylactic shock. Worsening of allergic symptoms during corticosteroid treatment may not always mean that the patient has failed treatment, although it may appear to be so.2,3 There are essentially four classes of corticosteroids: Class A, hydrocortisone-type, Class B, triamcinolone acetonide type, Class C, betamethasone type, and Class D, hydrocortisone-17-butyrate and clobetasone-17-butyrate type. Major* corticosteroids in Class A include cortisone, hydrocortisone, methylprednisolone, prednisolone, and prednisone. Major* corticosteroids in Class B include budesonide, fluocinolone, and triamcinolone. Major* corticosteroids in Class C include beclomethasone and dexamethasone. Finally, major* corticosteroids in Class D include betamethasone, fluticasone, and mometasone.4,5 Class D was later subdivided into Class D1 and D2 depending on the presence or 5,6 absence of a C16 methyl substitution and/or halogenation on C9 of the steroid B-ring. It is often hard to determine what exactly a patient is allergic to if they experience a reaction to a corticosteroid. -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM. -

Etats Rapides
List of European Pharmacopoeia Reference Standards Effective from 2015/12/24 Order Reference Standard Batch n° Quantity Sale Information Monograph Leaflet Storage Price Code per vial Unit Y0001756 Exemestane for system suitability 1 10 mg 1 2766 Yes +5°C ± 3°C 79 ! Y0001561 Abacavir sulfate 1 20 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001552 Abacavir for peak identification 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0001551 Abacavir for system suitability 1 10 mg 1 2589 Yes +5°C ± 3°C 79 ! Y0000055 Acamprosate calcium - reference spectrum 1 n/a 1 1585 79 ! Y0000116 Acamprosate impurity A 1 50 mg 1 3-aminopropane-1-sulphonic acid 1585 Yes +5°C ± 3°C 79 ! Y0000500 Acarbose 3 100 mg 1 See leaflet ; Batch 2 is valid until 31 August 2015 2089 Yes +5°C ± 3°C 79 ! Y0000354 Acarbose for identification 1 10 mg 1 2089 Yes +5°C ± 3°C 79 ! Y0000427 Acarbose for peak identification 3 20 mg 1 Batch 2 is valid until 31 January 2015 2089 Yes +5°C ± 3°C 79 ! A0040000 Acebutolol hydrochloride 1 50 mg 1 0871 Yes +5°C ± 3°C 79 ! Y0000359 Acebutolol impurity B 2 10 mg 1 -[3-acetyl-4-[(2RS)-2-hydroxy-3-[(1-methylethyl)amino] propoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! acetamide (diacetolol) Y0000127 Acebutolol impurity C 1 20 mg 1 N-(3-acetyl-4-hydroxyphenyl)butanamide 0871 Yes +5°C ± 3°C 79 ! Y0000128 Acebutolol impurity I 2 0.004 mg 1 N-[3-acetyl-4-[(2RS)-3-(ethylamino)-2-hydroxypropoxy]phenyl] 0871 Yes +5°C ± 3°C 79 ! butanamide Y0000056 Aceclofenac - reference spectrum 1 n/a 1 1281 79 ! Y0000085 Aceclofenac impurity F 2 15 mg 1 benzyl[[[2-[(2,6-dichlorophenyl)amino]phenyl]acetyl]oxy]acetate -

Inhaled Corticosteroids Asthma M E D I C I N E
LO N G -T E R M CONTROL INHALED CORTICOSTEROIDS ASTHMA M E D I C I N E When a person has asthma, How are Inhaled SOME BRAND NAMES OF the airways are very sensitive Corticosteroids taken? INHALED CORTICOSTEROIDS (Generic names in parentheses) to irritants and allergens. Inhaled corticosteroids are taken The inside walls of the airways Aerobid using an inhaler (or pump). Those (Flunisolide) tend to become swollen and brands that come in a metered dose covered with mucus, partially Azmacort inhaler should be used with a spacer. (Triamcinolone acetonide) blocking the flow of air into A spacer is a plastic tube or bag you and out of the lungs. Beclovent attach to your pump to help get the (Beclomethasone dipropionate) During an asthma episode, medicine to your airways. The few the swelling becomes worse Flovent brands that come in a dry powder (Fluticasone propionate) and more mucus is produced, inhaler do not require a spacer. Pulmicort making it very difficult to There is also one brand that can be (Budesonide) breathe. Inhaled corticosteroids used with a nebulizer machine. QVAR control the swelling and mucus (Beclomethasone dipropionate) production that make the Inhaled medicines go right to the Vanceril airways more sensitive and lungs and cause fewer side effects (Beclomethasone dipropionate) prevent asthma episodes. than medicine taken by mouth, like COMBINATION MEDICATION pills or liquid. Advair Diskus* Inhaled corticosteroids are (fluticasone propionate and the most effective long term salmeterol xinafoate) control asthma medicines. S i d e E f f e c t s : * Approved for children over 12 years old. -

Uncertainty Reigns in Error Disclosure Debate
74 Practice Trends S K I N & A L L E R G Y N E W S • August 2008 Uncertainty Reigns in Error Disclosure Debate B Y J A N E M . A N D E R S O N ington, Seattle, told attendees at the annual desire full disclosure of harmful errors, harmful to the patient,” Dr. Gallagher said. Contributing Writer meeting of the American College of Physi- while at the same time worrying that The University of Washington recently cians that physicians are unsure about what health care workers might hide them. In surveyed 4,000 physicians about commu- WA S H I N G T O N — Physicians generally to include when they disclose a medical er- disclosure, they want “an explicit state- nication with patients, colleagues, and believe that medical errors—especially ror. But he added that physicians are ac- ment that an error occurred,” details of health care institutions about errors. those that cause an adverse event—should tively debating the best way to proceed. what happened, and the implications for According to Dr. Gallagher, the survey be disclosed to patients, but there is dis- “Over the next 5 years, we’re going to see their health, he said. on error disclosure was sent to 2,000 physi- agreement about the level of detail that very exciting changes,” he said. “I think Physicians define errors more narrowly cians in Washington State and 2,000 Cana- should be provided, according to a physi- physicians as a profession will be leading than patients do. They agree in principle dian physicians. -

FLONASE (Fluticasone Propionate) Nasal Spray Bacterial, Viral, Or Parasitic Infection; Ocular Herpes Simplex)
HIGHLIGHTS OF PRESCRIBING INFORMATION • Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, These highlights do not include all the information needed to use contact dermatitis, rash) have been reported after administration of FLONASE safely and effectively. See full prescribing information for FLONASE nasal spray. Discontinue FLONASE nasal spray if such FLONASE. reactions occur. (5.3) • Potential worsening of infections (e.g., existing tuberculosis; fungal, FLONASE (fluticasone propionate) nasal spray bacterial, viral, or parasitic infection; ocular herpes simplex). Use with Initial U.S. Approval: 1994 caution in patients with these infections. More serious or even fatal course --------------------------- RECENT MAJOR CHANGES --------------------------- of chickenpox or measles can occur in susceptible patients. (5.4) • Hypercorticism and adrenal suppression may occur with very high Warnings and Precautions, Glaucoma and Cataracts (5.2) 1/2019 dosages or at the regular dosage in susceptible individuals. If such --------------------------- INDICATIONS AND USAGE ---------------------------- changes occur, discontinue FLONASE nasal spray slowly. (5.5) FLONASE nasal spray is a corticosteroid indicated for the management of the • Monitor growth of pediatric patients. (5.7) nasal symptoms of perennial nonallergic rhinitis in adult and pediatric patients ------------------------------ ADVERSE REACTIONS ------------------------------ aged 4 years and older. (1) The most common adverse reactions (>3%) are headache, pharyngitis, ----------------------- DOSAGE AND ADMINISTRATION ----------------------- epistaxis, nasal burning/nasal irritation, nausea/vomiting, asthma symptoms, For intranasal use only. Recommended starting dosages: and cough. (6.1) • Adults: 2 sprays per nostril once daily (200 mcg per day). (2.1) To report SUSPECTED ADVERSE REACTIONS, contact • Adolescents and children aged 4 years and older: 1 spray per nostril once GlaxoSmithKline at 1-888-825-5249 or FDA at 1-800-FDA-1088 or daily (100 mcg per day).