(12) Patent Application Publication (10) Pub. No.: US 2014/0302147 A1 HARTMAN Et Al
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
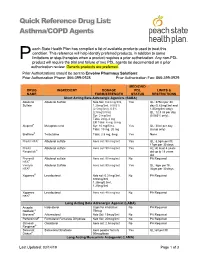
Asthma/COPD Agents
Quick Reference Drug List: Asthma/COPD Agents each State Health Plan has compiled a list of available products used to treat this condition. This reference will help identify preferred products, in addition to some P limitations or step-therapies when a product requires a prior authorization. Any non-PDL product will require the trial and failure of two PDL agents be documented on a prior authorization review. Generic products are preferred. Prior Authorizations should be sent to Envolve Pharmacy Solutions: Prior Authorization Phone: 866-399-0928 Prior Authorization Fax: 866-399-0929 MEDICAID DRUG INGREDIENT DOSAGE PDL LIMITS & NAME FORM/STRENGTH STATUS RESTRICTIONS Short Acting Beta Adrenergic Agonists (SABA) Albuterol Albuterol Sulfate Neb Sol: 0.63 mg/3ml, Yes QL: 375ml per 30 Sulfate 1.25mg/3ml, 0.083% day (0.63mg/3ml and (2.5mg/3ml), 0.5% 1/25mg/3ml only); (2.5mg/0.5ml) QL: 12.5 ml per day Syr: 2 mg/5ml (0.083% only); Tabs: 2mg, 4 mg ER Tabs: 4 mg, 8 mg Alupent® Metaproterenol Syr: 10 mg/5 mL Yes QL: 30ml per day Tabs: 10 mg, 20 mg (syrup only) Brethine® Terbutaline Tabs: 2.5 mg, 5mg Yes None ProAir HFA® Albuterol sulfate Aero sol: 90 mcg/act Yes QL: 8.5gm per fill, 17gm per 30 days ProAir Albuterol sulfate Aero sol: 90 mcg/act Yes AL: At least 4 years Respiclick® old up to 18 years old Proventil Albuterol sulfate Aero sol: 90 mcg/act No PA Required HFA® Ventolin Albuterol Sulfate Aero sol: 90 mcg/act Yes QL: 8gm per fill, HFA® 36gm per 30 days Xopenex® Levalbuterol Neb sol: 0.31mg/3ml, No PA Required 0.63mg/3ml, 1.25mg/0.5ml, 1.25mg/3ml -

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Spirometry Protocol and Software Training Handout
SPIROMETRY PROTOCOL AND SOFTWARE TRAINING HANDOUT Spirometry Protocol Key Points in Performing Spirometry ▪ Always demonstrate the maneuver ▪ Prompt the participant to BLAST out the air ▪ Continue by having the participant PUSH out the air ▪ Continue until the balloon bursts or at least six seconds Getting ready to do Spirometry Subjects will be instructed to not use any bronchodilator medicines for at least 4 hours prior to the appointment (in the case of salmeterol [Serevent] or Fomoterol (foradil)or Tritoprium (Spiriva) avoid for 12 hours prior to the test). Bronchodilator medicines include: Short acting bronchodilators Albuterol (ventolin, proventil, airet, volmax tablet) Levalbuterol (xopenex) Metaproterenol (alupent) Pirbuterol (maxair) Terbutaline (brethaire, brethine, bricanyl) Isoetharine (bronkosol) Ipratropium (atrovent) Ipratropium and albuterol combination (Combivent) Isoproteranol (isuprel) Primatine 1 CHW Educational Protocols – Spirometry Protocol and Software Training Handout Bitolterol (tornalate) Long acting bronchodilator Salmeterol (serevent) Fomoterol (foradil) Tritoprium (Spiriva) Bronchodilators should be avoided because they can affect the spirometry results. If the patient is having symptoms from asthma and needs to use the medicine, ask that the medicine be used 4 hours before the visit, so that the next dose is due during the visit. Patients can then do the spirometry and then the patient can take the medicine after the test is completed. When scheduling the follow-up spirometry visit, if at all possible, try to make the visit at the same time as the initial baseline visit. 1. Preparing the Computer 1. Check to see if the time and date on the lower right hand corner is current. If the date and time is NOT, the spirometer will NOT sync with the computer or the Easy One software. -

Separation of Β-Receptor Blockers and Analogs by Capillary Liquid Chromatography
ORIGINAL ARTICLES College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, China Separation of b-receptor blockers and analogs by Capillary Liquid Chromatography (CLC) and Pressurized Capillary Electrochromatography (pCEC) using a vancomycin chiral stationary phase column Zhongyi Chen, Su Zeng, Tongwei Yao Received September 15, 2006, accepted October 28, 2006 Tongwei Jao, Department of Pharmaceutical Analysis and Drug Metabolism, College of Pharmaceutical Sciences, Zhejiang University, Hangzhou, 310031, China [email protected] Pharmazie 62: 585–592 (2007) doi: 10.1691/ph.2007.8.6194 Enantiomeric separation of chiral pharmaceuticals was carried out by means of in capillary liquid chroma- tography (CLC) and pressurized capillary electrochromatography (pCEC) using a vancomycin chiral sta- tionary phase (CSP). A 100 mm I.D. fused-silica capillary was packed with 5 mm diameter silica particles modified with vancomycin. Enantiomeric resolution of fifteen b-receptor blockers and analogs was stu- died by polar organic CLC mode and reversed-phase pCEC mode using mobile phases containing methanol-isopropanol-acetic acid-triethylamine and TEAA buffer-methanol, respectively. Several factors affecting chiral separation were investigated in both CLC and pCEC mode. Good enantiomeric resolution was achieved by CLC mode for propranolol, celiprolol, esmolol, bisoprolol, atenolol, metoprolol and car- teolol using methanol-isopropanol-acetic acid-triethylamine (70 : 30 : 0.05 : 0.05, v/v/v/v) as mobile phase and for clenbuterol, bambuterol, terbutaline, and salbutamol using methanol-isopropanol-acetic acid- triethylamine (50 : 50 : 0.05 : 005 or 50 : 50: 0.025 : 0.05, v/v/v/v) as mobile phase. The baseline was achieved by pCEC mode for the separation of esmolol, bisoprolol, atenolol, metoprolol, carteolol in the mobile phase containing MeOH-0.05%TEAA (pH 7.0) (90 : 10, v/v) (–10 kV), and that of propranolol and celiprolol in the mobile phase containing MeOH-0.025%TEAA (pH 7.0) (90 : 10, v/v)(–10 kV). -

Clenbuterol Human Effects the Effect of Clenbuterol in Humans Is Researched Through Examining the History and Regulations of the Drug
Clenbuterol Human Effects The effect of clenbuterol in humans is researched through examining the history and regulations of the drug. Specifically, Alberto Contador’s case is considered. Tag Words: Clenbuterol; drugs; Beta-2 Agonist; Effects; Thermogenic; Fat; Harmful; Authors: Jessie Yeh, Horace Lau, Danielle Lovisone with Julie M. Fagan, Ph.D. Summary (written by Danielle Lovisone) As a sympathomimetic and Beta-2 agonist, clenbuterol have several deleterious effects on the human body. The drug acts as a thermogenic stimulant, increasing lean muscle mass and respiratory efficiency while reducing fat. Cases on clenbuterol, including animal tests and human occurrences, support these unnatural and potentially harmful effects. With this, athletes and body builders have recently increased their use of the drug. Particularly, Alberto Contador has recently been targeted for having traces of clenbuterol in a urine drug test. Contador claims, instead of doping, this trace amount was unknowingly received from ingesting beef in Spain during the 2010 Tour de France. Although clenbuterol is banned in most areas of the world, this explanation seems plausible because the drug is poorly regulated by organizations such as the FDA. To examine Contador’s case further, our group compiled research on clenbuterol to ultimately hypothesize that Contador received this trace amount from contaminated beef. Our findings were submitted to the World Anti-Doping Agency as part of our Service Project. Video Link Class project 2010 fall: www.youtube.com/watch?v=ZTeJiBvbLhA The Issue: Clenbuterol The Effects of Clenbuterol on the Human Body By Jessie Yeh What is Clenbuterol? Clenbuterol is a chemical compound closely resembling the structure of an amine. -

Validation of New Analytical Methodology for Determining Fenoterolhydrobromide by HPLC: Application in Pharmaceutical Products
Artigo Original/Original Article Validation of new analytical methodology for determining fenoterolhydrobromide by HPLC: application in pharmaceutical products Validação de uma nova metodologia analítica para determinação de bromidrato de fenoterol por CLAE: aplicações em produtos farmacêuticos RIALA6/1475 *Helena Miyoco YANO1, Fernanda Fernandes FARIAS1, Marcelo Beiriz DEL BIANCO1, Pedro Lopez GARCIA2 *Endereço para correspondência: 1Núcleo de Ensaios Físicos e Químicos em Medicamentos, Centro de Medicamentos, Cosméticos e Saneantes, Instituto Adolfo Lutz, Av. Doutor Arnaldo, 355, CEP: 01246-902, São Paulo, SP, Brasil. E-mail: [email protected] 2Departamento de Farmácia, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, SP, Brasil. Recebido: 31.10.2011- Aceito para publicação: 20.04.2012 RESUMO Bromidrato de fenoterol é um agente agonista adrenérgico β2-seletivo utilizado para tratamento de asma e doenças pulmonares crônicas obstrutivas. A metodologia analítica por cromatografia líquida de alta eficiência (CLAE) foi desenvolvida e validada para a determinação quantitativa de bromidrato de fenoterol. A condição analítica empregada incluiu coluna em fase reversa C18 (150 mm × 3,9 mm d.i., 5 µm) Thermo®, fase móvel composta de mistura de acetonitrila e água (30:70, v/v) com 0,1% de trietilamina e pH ajustado para 5,0 com ácido fórmico, vazão de 1,0 mL.min-1 e detecção em UV a 276 nm. A faixa de linearidade foi de 0,025 a 0,15 mg.mL-1; a curva analítica mostrou coeficiente de correlação > 0,999. O limite de detecção (LD) foi de 0,003 mg.mL-1 e o limite de quantificação de 0,012 mg.mL-1. -

Ep 2560611 B1
(19) TZZ Z___T (11) EP 2 560 611 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) 03.01.2018 Bulletin 2018/01 (86) International application number: (21) Application number: 11719211.2 PCT/EP2011/056227 (22) Date of filing: 19.04.2011 (87) International publication number: WO 2011/131663 (27.10.2011 Gazette 2011/43) (54) "PROCESS FOR PROVIDING PARTICLES WITH REDUCED ELECTROSTATIC CHARGES" VERFAHREN ZUR BEREITSTELLUNG VON PARTIKELN MIT REDUZIERTEN ELEKTROSTATISCHEN LADUNGEN PROCÉDÉ DE PRÉPARATION DE PARTICULES AYANT DES CHARGES ÉLECTROSTATIQUES RÉDUITES (84) Designated Contracting States: • GUCHARDI ET AL: "Influence of fine lactose and AL AT BE BG CH CY CZ DE DK EE ES FI FR GB magnesium stearate on low dose dry powder GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO inhaler formulations", INTERNATIONAL PL PT RO RS SE SI SK SM TR JOURNAL OF PHARMACEUTICS, ELSEVIER BV, NL LNKD- DOI:10.1016/J.IJPHARM.2007.06.041, (30) Priority: 21.04.2010 EP 10160565 vol. 348, no. 1-2, 19 December 2007 (2007-12-19), pages 10-17, XP022393884, ISSN: 0378-5173 (43) Date of publication of application: • ELAJNAF A ET AL: "Electrostatic 27.02.2013 Bulletin 2013/09 characterisation of inhaled powders: Effect of contact surface and relative humidity", (73) Proprietor: Chiesi Farmaceutici S.p.A. EUROPEAN JOURNAL OF PHARMACEUTICAL 43100 Parma (IT) SCIENCES, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/J.EJPS.2006.07.006, vol. 29, no. 5, 1 (72) Inventors: December 2006 (2006-12-01), pages 375-384, • COCCONI, Daniela XP025137181, ISSN: 0928-0987 [retrieved on I-43100 Parma (IT) 2006-12-01] • MUSA, Rossella • CHAN ET AL: "Dry powder aerosol drug I-43100 Parma (IT) delivery-Opportunities for colloid and surface scientists", COLLOIDS AND SURFACES. -

Difference Between Patient-Reported Side Effects of Ciclesonide Versus fluticasone Propionate
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Respiratory Medicine (2010) 104, 1825e1833 available at www.sciencedirect.com journal homepage: www.elsevier.com/locate/rmed Difference between patient-reported side effects of ciclesonide versus fluticasone propionate Thys van der Molen a, Juliet M. Foster a,b, Manfred Caeser c,e, Thomas Mu¨ller c, Dirkje S. Postma d,* a Department of General Practice, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands b Woolcock Institute of Medical Research, 431 Glebe Point Rd, Glebe NSW 2037, Sydney, Australia c Nycomed GmbH, Byk-Gulden-Straße 2, 78467 Konstanz, Konstanz, Germany d Department of Pulmonary Diseases, University Medical Center Groningen, University of Groningen, Hanzeplein 1, 9700 RB, Groningen, The Netherlands Received 18 January 2010; accepted 26 May 2010 Available online 2 July 2010 KEYWORDS Summary Adverse events; Rationale: Patient-reported outcomes provide new insights into the dynamics of asthma Inhaled corticosteroid management. Further to asthma control and quality of life, self-reported side effects of treat- questionnaire; ment can be assessed with the validated Inhaled Corticosteroid Questionnaire (ICQ). ICQ; Objectives: To compare patient-reported side effects between the inhaled corticosteroids Patient-reported ciclesonide and fluticasone propionate. outcomes Methods: Patients with moderate or moderate-to-severe asthma, pre-treated with a constant dose and type of medication, were randomized in three separate studies: 1) once daily cicle- sonide 320 mg(n Z 234) or twice daily fluticasone propionate 200 mg(n Z 240); 2) twice daily ciclesonide 320 mg(n Z 255) or twice daily fluticasone propionate 375 mg(n Z 273); and 3) twice daily ciclesonide 320 mg(n Z 259) or twice daily fluticasone propionate 500 mg (n Z 244). -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

COVID-19 Evidence Bulletin 8
COVID-19 Evidence Bulletin 8 Public Health England PHE International Epidemiology Daily Evidence Digest – 22nd April 2020 – 21st April 2020 – 20th April 2020 NICE COVID-19 rapid guideline: acute myocardial injury [NG171] Published 23rd April The purpose of this guideline is to help healthcare professionals who are not cardiology specialists identify and treat acute myocardial injury and its cardiac complications in adults with known or suspected COVID-19 but without known pre-existing cardiovascular disease. COVID-19 rapid guideline: gastrointestinal and liver conditions treated with drugs affecting the immune response [NG172] Published 23rd April The purpose of this guideline is to maximise the safety of children and adults who have gastrointestinal or liver conditions treated with drugs affecting the immune response during the COVID 19 pandemic. It also aims to protect staff from infection and enable services to make the best use of NHS resources. COVID-19 rapid guideline: managing symptoms (including at the end of life) in the community Published 3rd April, Last updated 22nd April NHS England Specialty Guides: Clinical guide for acute kidney injury in hospitalised patients with COVID-19 outside the intensive care unit during the coronavirus pandemic (22nd April - updated) Management of palliative care in hospital during the coronavirus pandemic (22nd April – updated) Department of Health and Social Care Medicines that cannot be parallel exported from the UK (22nd April) 33 medicines have been added to the parallel export list and the -

The Inhaled Steroid Ciclesonide Blocks SARS-Cov-2 RNA Replication by Targeting Viral
bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 1 The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting viral 2 replication-transcription complex in culture cells 3 4 Shutoku Matsuyamaa#, Miyuki Kawasea, Naganori Naoa, Kazuya Shiratoa, Makoto Ujikeb, Wataru 5 Kamitanic, Masayuki Shimojimad, and Shuetsu Fukushid 6 7 aDepartment of Virology III, National Institute of Infectious Diseases, Tokyo, Japan 8 bFaculty of Veterinary Medicine, Nippon Veterinary and Life Science University, Tokyo, Japan 9 cDepartment of Infectious Diseases and Host Defense, Gunma University Graduate School of 10 Medicine, Gunma, Japan 11 dDepartment of Virology I, National Institute of Infectious Diseases, Tokyo, Japan. 12 13 Running Head: Ciclesonide blocks SARS-CoV-2 replication 14 15 #Address correspondence to Shutoku Matsuyama, [email protected] 16 17 Word count: Abstract 149, Text 3,016 bioRxiv preprint doi: https://doi.org/10.1101/2020.08.22.258459; this version posted August 24, 2020. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. 18 Abstract 19 We screened steroid compounds to obtain a drug expected to block host inflammatory responses and 20 MERS-CoV replication. Ciclesonide, an inhaled corticosteroid, suppressed replication of MERS-CoV 21 and other coronaviruses, including SARS-CoV-2, the cause of COVID-19, in cultured cells. The 22 effective concentration (EC90) of ciclesonide for SARS-CoV-2 in differentiated human bronchial 23 tracheal epithelial cells was 0.55 μM.