5 August 2004 Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Validation of New Analytical Methodology for Determining Fenoterolhydrobromide by HPLC: Application in Pharmaceutical Products
Artigo Original/Original Article Validation of new analytical methodology for determining fenoterolhydrobromide by HPLC: application in pharmaceutical products Validação de uma nova metodologia analítica para determinação de bromidrato de fenoterol por CLAE: aplicações em produtos farmacêuticos RIALA6/1475 *Helena Miyoco YANO1, Fernanda Fernandes FARIAS1, Marcelo Beiriz DEL BIANCO1, Pedro Lopez GARCIA2 *Endereço para correspondência: 1Núcleo de Ensaios Físicos e Químicos em Medicamentos, Centro de Medicamentos, Cosméticos e Saneantes, Instituto Adolfo Lutz, Av. Doutor Arnaldo, 355, CEP: 01246-902, São Paulo, SP, Brasil. E-mail: [email protected] 2Departamento de Farmácia, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, SP, Brasil. Recebido: 31.10.2011- Aceito para publicação: 20.04.2012 RESUMO Bromidrato de fenoterol é um agente agonista adrenérgico β2-seletivo utilizado para tratamento de asma e doenças pulmonares crônicas obstrutivas. A metodologia analítica por cromatografia líquida de alta eficiência (CLAE) foi desenvolvida e validada para a determinação quantitativa de bromidrato de fenoterol. A condição analítica empregada incluiu coluna em fase reversa C18 (150 mm × 3,9 mm d.i., 5 µm) Thermo®, fase móvel composta de mistura de acetonitrila e água (30:70, v/v) com 0,1% de trietilamina e pH ajustado para 5,0 com ácido fórmico, vazão de 1,0 mL.min-1 e detecção em UV a 276 nm. A faixa de linearidade foi de 0,025 a 0,15 mg.mL-1; a curva analítica mostrou coeficiente de correlação > 0,999. O limite de detecção (LD) foi de 0,003 mg.mL-1 e o limite de quantificação de 0,012 mg.mL-1. -

Ep 2560611 B1
(19) TZZ Z___T (11) EP 2 560 611 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) 03.01.2018 Bulletin 2018/01 (86) International application number: (21) Application number: 11719211.2 PCT/EP2011/056227 (22) Date of filing: 19.04.2011 (87) International publication number: WO 2011/131663 (27.10.2011 Gazette 2011/43) (54) "PROCESS FOR PROVIDING PARTICLES WITH REDUCED ELECTROSTATIC CHARGES" VERFAHREN ZUR BEREITSTELLUNG VON PARTIKELN MIT REDUZIERTEN ELEKTROSTATISCHEN LADUNGEN PROCÉDÉ DE PRÉPARATION DE PARTICULES AYANT DES CHARGES ÉLECTROSTATIQUES RÉDUITES (84) Designated Contracting States: • GUCHARDI ET AL: "Influence of fine lactose and AL AT BE BG CH CY CZ DE DK EE ES FI FR GB magnesium stearate on low dose dry powder GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO inhaler formulations", INTERNATIONAL PL PT RO RS SE SI SK SM TR JOURNAL OF PHARMACEUTICS, ELSEVIER BV, NL LNKD- DOI:10.1016/J.IJPHARM.2007.06.041, (30) Priority: 21.04.2010 EP 10160565 vol. 348, no. 1-2, 19 December 2007 (2007-12-19), pages 10-17, XP022393884, ISSN: 0378-5173 (43) Date of publication of application: • ELAJNAF A ET AL: "Electrostatic 27.02.2013 Bulletin 2013/09 characterisation of inhaled powders: Effect of contact surface and relative humidity", (73) Proprietor: Chiesi Farmaceutici S.p.A. EUROPEAN JOURNAL OF PHARMACEUTICAL 43100 Parma (IT) SCIENCES, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/J.EJPS.2006.07.006, vol. 29, no. 5, 1 (72) Inventors: December 2006 (2006-12-01), pages 375-384, • COCCONI, Daniela XP025137181, ISSN: 0928-0987 [retrieved on I-43100 Parma (IT) 2006-12-01] • MUSA, Rossella • CHAN ET AL: "Dry powder aerosol drug I-43100 Parma (IT) delivery-Opportunities for colloid and surface scientists", COLLOIDS AND SURFACES. -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -
Ep 2626065 A1
(19) TZZ Z_T (11) EP 2 626 065 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: A61K 31/137 (2006.01) A61K 31/135 (2006.01) 14.08.2013 Bulletin 2013/33 A61K 31/4704 (2006.01) A61K 31/58 (2006.01) A61K 31/56 (2006.01) A61K 9/12 (2006.01) (2006.01) (2006.01) (21) Application number: 11827927.2 A61K 9/14 A61P 11/06 (86) International application number: Date of filing: 01.02.2011 (22) PCT/CN2011/070883 (87) International publication number: WO 2012/041031 (05.04.2012 Gazette 2012/14) (84) Designated Contracting States: (72) Inventor: WU, Wei-hsiu AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Taipei GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Taiwan (TW) PL PT RO RS SE SI SK SM TR (74) Representative: Patentanwaltskanzlei WILHELM (30) Priority: 28.09.2010 CN 201010502339 & BECK Prinzenstrasse 13 (71) Applicant: Intech Biopharm Ltd. 80639 München (DE) Taipei (TW) (54) COMPOUND COMPOSITION FOR INHALATION USED FOR TREATING ASTHMA (57) An inhaled pharmaceutical composition con- tric way as a controller. The eccentric way control therapy tains primary active ingredients of beta2- agonist and cor- could create a low blood concentration period during the ticosteroids.The pharmaceuticalcompositions disclosed day and minimize the acute tolerance phenomenon (or in the present invention are to be inhaled by a patient so called tachyphylaxis) for bronchodilator - beta2-ago- when needed as a reliever, or administrated in an eccen- nists in treating asthma or other obstructive respiratory disorders. -
ATC/DDD Classification
WHO Drug Information Vol. 27, No. 2, 2013 ATC/DDD Classification ATC/DDD Classification (Temporary) The following anatomical therapeutic codes (ATC), defined daily doses (DDD) and alterations were considered by the WHO International Working Group for Drug Statistics Methodology at its meeting in March 2013. Comments or objections to the decisions should be forwarded to the WHO Collaborating Centre for Drug Statistics Methodology at [email protected]. The new ATC codes, DDDs and alterations will then be considered final and be included in the January 2014 version of the ATC/DDD index. The inclusion of a substance in the lists does not imply any recommendation for use in medicine or pharmacy. New ATC 5th level codes: ATC level INN/Common name ATC code afamelanotide D02BB02 apremilast L04AA32 brimonidine D11AX21 calcium citrate A12AA13 carfilzomib L01XX45 colestilan V03AE06 delamanid G04AK06 dienogest and ethinylestradiol G03AA16 eliglustat A16AX10 empagliflozin A10BX12 encephalitis, Japanese, live attenuated J07BA03 formoterol and fluticasone R03AK11 insulin degludec A10AE06 insulin degludec and insulin aspart A10AD05 lamivudine, tenofovir disoproxil and efavirenz J05AR11 macitentan C02KX04 metformin and dapagliflozin A10BD15 nalmefene N07BB05 naloxegol A06AH03 nomegestrol and estrogen G03FB12 obinutuzumab L01XC15 ocriplasmin S01AX22 ospemifene G03XC05 Continued/ 130 WHO Drug Information Vol. 27, No. 2, 2013 ATC/DDD Classification ATC level INN/Common name ATC code pomalidomide L04AX06 serelaxin C01DX21 strontium ranelate and colecalciferol -
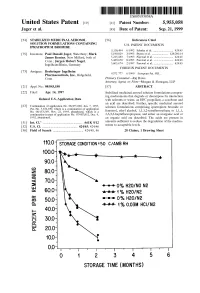
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int. -

WO 2014/141069 Al 18 September 2014 (18.09.2014) P O P C T
(12) INTERNATIONAL APPLICATION PUBLISHED UNDER THE PATENT COOPERATION TREATY (PCT) (19) World Intellectual Property Organization International Bureau (10) International Publication Number (43) International Publication Date WO 2014/141069 Al 18 September 2014 (18.09.2014) P O P C T (51) International Patent Classification: Novartis Pharmaceuticals Corporation, 150 Industrial A61K 9/00 (2006.01) A61K 31/00 (2006.01) Road, San Carlos, California 94070 (US). A61K 9/16 (2006.01) (74) Agent: MAZZA, Michael; Novartis Vaccines and Dia (21) International Application Number: gnostics, Inc., 4560 Horton Street, Emeryville, California PCT/IB2014/059632 94608 (US). (22) International Filing Date: (81) Designated States (unless otherwise indicated, for every 11 March 2014 ( 11.03.2014) kind of national protection available): AE, AG, AL, AM, AO, AT, AU, AZ, BA, BB, BG, BH, BN, BR, BW, BY, (25) Filing Language: English BZ, CA, CH, CL, CN, CO, CR, CU, CZ, DE, DK, DM, (26) Publication Language: English DO, DZ, EC, EE, EG, ES, FI, GB, GD, GE, GH, GM, GT, HN, HR, HU, ID, IL, IN, IR, IS, JP, KE, KG, KN, KP, KR, (30) Priority Data: KZ, LA, LC, LK, LR, LS, LT, LU, LY, MA, MD, ME, 61/784,865 14 March 2013 (14.03.2013) US MG, MK, MN, MW, MX, MY, MZ, NA, NG, NI, NO, NZ, (71) Applicant (for all designated States except US) : NO¬ OM, PA, PE, PG, PH, PL, PT, QA, RO, RS, RU, RW, SA, VARTIS AG [CH/CH]; Lichtstrasse 35, CH-4056 Basel SC, SD, SE, SG, SK, SL, SM, ST, SV, SY, TH, TJ, TM, (CH). -

Vr Meds Ex01 3B 0825S Coding Manual Supplement Page 1
vr_meds_ex01_3b_0825s Coding Manual Supplement MEDNAME OTHER_CODE ATC_CODE SYSTEM THER_GP PHRM_GP CHEM_GP SODIUM FLUORIDE A12CD01 A01AA01 A A01 A01A A01AA SODIUM MONOFLUOROPHOSPHATE A12CD02 A01AA02 A A01 A01A A01AA HYDROGEN PEROXIDE D08AX01 A01AB02 A A01 A01A A01AB HYDROGEN PEROXIDE S02AA06 A01AB02 A A01 A01A A01AB CHLORHEXIDINE B05CA02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D08AC02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D09AA12 A01AB03 A A01 A01A A01AB CHLORHEXIDINE R02AA05 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S01AX09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S02AA09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S03AA04 A01AB03 A A01 A01A A01AB AMPHOTERICIN B A07AA07 A01AB04 A A01 A01A A01AB AMPHOTERICIN B G01AA03 A01AB04 A A01 A01A A01AB AMPHOTERICIN B J02AA01 A01AB04 A A01 A01A A01AB POLYNOXYLIN D01AE05 A01AB05 A A01 A01A A01AB OXYQUINOLINE D08AH03 A01AB07 A A01 A01A A01AB OXYQUINOLINE G01AC30 A01AB07 A A01 A01A A01AB OXYQUINOLINE R02AA14 A01AB07 A A01 A01A A01AB NEOMYCIN A07AA01 A01AB08 A A01 A01A A01AB NEOMYCIN B05CA09 A01AB08 A A01 A01A A01AB NEOMYCIN D06AX04 A01AB08 A A01 A01A A01AB NEOMYCIN J01GB05 A01AB08 A A01 A01A A01AB NEOMYCIN R02AB01 A01AB08 A A01 A01A A01AB NEOMYCIN S01AA03 A01AB08 A A01 A01A A01AB NEOMYCIN S02AA07 A01AB08 A A01 A01A A01AB NEOMYCIN S03AA01 A01AB08 A A01 A01A A01AB MICONAZOLE A07AC01 A01AB09 A A01 A01A A01AB MICONAZOLE D01AC02 A01AB09 A A01 A01A A01AB MICONAZOLE G01AF04 A01AB09 A A01 A01A A01AB MICONAZOLE J02AB01 A01AB09 A A01 A01A A01AB MICONAZOLE S02AA13 A01AB09 A A01 A01A A01AB NATAMYCIN A07AA03 A01AB10 A A01 -

(12) Patent Application Publication (10) Pub. No.: US 2009/0047336A1 Yang Et Al
US 20090047336A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2009/0047336A1 Yang et al. (43) Pub. Date: Feb. 19, 2009 (54) NOVEL FORMULATION OF DEHYDRATED Publication Classification LPID VESICLES FOR CONTROLLED (51) Int. Cl. RELEASE OF ACTIVE PHARMACEUTICAL A63/37 (2006.01) INGREDIENT VLANHALATION A6II 47/06 (2006.01) A 6LX 9/27 (2006.01) (75) Inventors: Zhijun Yang, Kowloon (CN); A6IR 9/14 (2006.01) Wenhua Huang, Kowloon (CN); A6IP II/00 (2006.01) Chi Sun Wong, Hong Kong (CN); A6IP II/06 (2006.01) Zhongzhen Zhao, Kowloon (CN) (52) U.S. Cl. .......... 424/450; 424/489: 514/653: 514/772 Correspondence Address: (57) ABSTRACT LEYDIG VOIT & MAYER, LTD A new formulation of dehydrated lipid vesicles employs a 700 THIRTEENTH ST. NW, SUITE 300 vesicle preserver and permits the control of release and deliv WASHINGTON, DC 20005-3960 (US) ery of active pharmaceutical ingredients into the respiratory system for treatment in particular of asthma. The typical (73) Assignee: HONG KONG BAPTIST formulation provides controlled release of the active pharma UNIVERSITY, Kowloon (CN) ceutical ingredient from 0% to 100% from 0 to 72 hours after inhalation, changes the systemic administration to topical (21) Appl. No.: 11/840,537 administration, allows prolonged therapeutic period for one administration, increased stability, with reduced dose, (22) Filed: Aug. 17, 2007 reduced systemic side effects, reduced toxicity. S Patent Application Publication US 2009/0047336A1 60 T. 50 40 30 20 O O Time (hours) --DOPC+1% gly cerin w8m DOTAP+1% glycerin FIG. 2 US 2009/0047336A1 Feb. 19, 2009 NOVEL FORMULATION OF DEHYDRATED blood pressure. -

Management of Asthma in Athletes Michael G
Journal of Athletic Training 2005;40(3):224±245 q by the National Athletic Trainers' Association, Inc www.journalofathletictraining.org National Athletic Trainers' Association Position Statement: Management of Asthma in Athletes Michael G. Miller*; John M. Weiler²; Robert Baker³; James Collins§; Gilbert D'Alonzo\ *Western Michigan University, Kalamazoo, MI; ²University of Iowa and CompleWare, Iowa City, IA; ³Michigan State University Kalamazoo Center for Medical Studies, Kalamazoo, MI; §San Diego Chargers, San Diego, CA; \Temple University School of Medicine, Philadelphia, PA Michael G. Miller, EdD, ATC, CSCS; John M. Weiler, MD; Robert Baker, MD, PhD, ATC; James Collins, ATC; and Gilbert D'Alonzo, DO, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and ®nal approval of the article. Address correspondence to National Athletic Trainers' Association, Communications Department, 2952 Stemmons Freeway, Dallas, TX 75247. Objective: To present guidelines for the recognition, prophy- in supervising therapies to prevent and control asthma symp- laxis, and management of asthma that lead to improvement in toms. It is also important for the athletic trainer to recognize the quality of care certi®ed athletic trainers and other heath care when asthma is not the underlying cause for respiratory dif®- providers can offer to athletes with asthma, especially exercise- culties, so that the athlete can be evaluated and treated prop- induced asthma. erly. Background: Many athletes have dif®culty breathing during Recommendations: The recommendations contained in this or after athletic events and practices. Although a wide variety position statement describe a structured approach for the di- of conditions can predispose an athlete to breathing dif®culties, agnosis and management of asthma in an exercising popula- tion. -

Short-Acting Inhaled B2-Agonists: Why, Whom, What, How?
REVIEW Andrzej Emeryk1, Justyna Emeryk-Maksymiuk2 1Department of Pediatric Pulmonology and Rheumatology Medical University in Lublin, Poland 2Chair of Internal Medicine and Department of Internal Medicine in Nursing Medical University in Lublin, Poland Short-acting inhaled b2-agonists: why, whom, what, how? Abstract We showed the present data about the efficacy and safety of inhaled short-acting b2-agonists (SABA), such as salbutamol and fenoterol, in the management of obstructive diseases in children and adults. Our work discusses major mechanisms of action, clinical effects, possible side effects and indications of inhaled SABA. We presented current recommendations for the position of SABA in the therapy of obstructive diseases in children and adults, particularly in asthma and chronic obstructive pulmonary disease. Key words: short-acting b2-agonist, salbutamol, fenoterol, inhalation, nebulization, asthma, COPD Adv Respir Med. 2020; 88: 443–449 Introduction after administration of inhaled salbutamol, but the duration of action does not exceed 4–6 hours Short-acting b2-agonists (SABA) stimulate (Figure 1) [8]. b2-adrenergic receptor (b2-AR). They are called Drugs from this group provide effective b2-mimetics or b2-agonists. First selective SABA protection against exercise-induced bronchoc- widely used in clinical practice appeared more onstriction within 0.5–2.0 hours also against than 50 years ago. They were introduced to the bronchoconstriction triggered by exposure to global market in the following order: terbutaline sensitizing allergen [9, 10]. Clinical studies show (1966 yr.), salbutamol (1968 yr.) and fenoterol more potent bronchodilation and less side effects (1970 yr.) [1]. Next drugs, such as levalbuterol, of R-salbutamol in comparison with racemic sal- reproterol, rimiterol, klenbuterol and pirbuterol, butamol [11–13]. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0302147 A1 HARTMAN Et Al
US 20140302147A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0302147 A1 HARTMAN et al. (43) Pub. Date: Oct. 9, 2014 (54) RESPRABLEAGGLOMERATES OF POROUS Publication Classification CARRIER PARTICLES AND MICRONIZED DRUG (51) Int. Cl. A619/16 (2006.01) (71) Applicants: Michael HARTMAN, Millbrae, CA A619/00 (2006.01) (US); Thomas ETARARA, A613 L/40 (2006.01) Burlingame, CA (US); Patrick TEUNG, A613 L/4704 (2006.01) Sunnyvale, CA (US); Jeffry G WEERS, A613 L/58 (2006.01) Belmont, CA (US) (52) U.S. Cl. CPC ........... A61 K9/1617 (2013.01); A61 K3I/4704 (72) Inventors: Michael HARTMAN, Millbrae, CA (2013.01); A61 K3I/58 (2013.01); A61K 31/40 (US); Thomas ETARARA, (2013.01); A61 K9/1682 (2013.01); A61 K Burlingame, CA (US); Patrick TEUNG, 9/0075 (2013.01) Sunnyvale, CA (US); Jeffry G WEERS, USPC ............................ 424/489: 514/312: 514/171 Belmont, CA (US) (57) ABSTRACT Embodiments of the present invention provide a dry powder Assignee: composition comprising porous carrier particles associated (73) NOVARTIS AG, Basel (CH) with one, two, three or more micronized drugs or APIs wherein an ordered mixture between the micronized drug or (21) Appl. No.: 14/202,262 drugs and the carrier particle results, such that the micronized drug or drugs adhere strongly to the carrier particles forming a stable ordered mixture of respirable agglomerates. Embodi (22) Filed: Mar 10, 2014 ments of the present invention further comprise a spray-dry ing process to create the respirable agglomerates. Embodi ments of the present invention further relate to the use of the Related U.S.