ATC/DDD Classification
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on Pulmonary Hypertension 2015 (TF08) - Task Force Members and Additional Contributors
Guidelines on Pulmonary Hypertension 2015 (TF08) - Task Force Members and Additional Contributors For ESC Guidelines: The report below lists declarations of interest as reported to the ESC by the experts covering the period of the Guidelines production, from Task Force creation to publication. Expert Type of Relationship with Industry Beghetti Maurice A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Novartis : Pulmonary hypertension imatinib (2012) - Pfizer : Pulmonary hypertension sildenafil (2012-2013) - Bayer Schering Pharma : Pulmonary hypertension riociguat (2012-2013-2014-2015) - Eli Lilly : Pulmonary hypertension tadalafil (2012-2013-2014-2015) - Actelion : Pulmonary hypertension, Tracleer, Macitentan, Selexipag (2012-2013-2014-2015) - GlaxoSmithKline : pulmonary hypertension Ambrisentan (2012-2015) - Novartis : Pulmonary hypertension riociguat (2013) - GlaxoSmithKline : ambrisentan (2014) D - Research funding (departmental or institutional). - Actelion : no relation to a specific product (2012-2013) - Bayer Schering Pharma : no relation to a specific product (2014-2015) Galie Nazzareno A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Eli Lilly : pulmonary hypertension (2012-2013) - Novartis : pulmonary hypertension (2012-2013) - Pfizer : pulmonary hypertension (2012-2013) - Actelion : pulmonary hypertension (2012-2013) - GlaxoSmithKline : pulmonary hypertension (2012-2013) -

Validation of New Analytical Methodology for Determining Fenoterolhydrobromide by HPLC: Application in Pharmaceutical Products
Artigo Original/Original Article Validation of new analytical methodology for determining fenoterolhydrobromide by HPLC: application in pharmaceutical products Validação de uma nova metodologia analítica para determinação de bromidrato de fenoterol por CLAE: aplicações em produtos farmacêuticos RIALA6/1475 *Helena Miyoco YANO1, Fernanda Fernandes FARIAS1, Marcelo Beiriz DEL BIANCO1, Pedro Lopez GARCIA2 *Endereço para correspondência: 1Núcleo de Ensaios Físicos e Químicos em Medicamentos, Centro de Medicamentos, Cosméticos e Saneantes, Instituto Adolfo Lutz, Av. Doutor Arnaldo, 355, CEP: 01246-902, São Paulo, SP, Brasil. E-mail: [email protected] 2Departamento de Farmácia, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, SP, Brasil. Recebido: 31.10.2011- Aceito para publicação: 20.04.2012 RESUMO Bromidrato de fenoterol é um agente agonista adrenérgico β2-seletivo utilizado para tratamento de asma e doenças pulmonares crônicas obstrutivas. A metodologia analítica por cromatografia líquida de alta eficiência (CLAE) foi desenvolvida e validada para a determinação quantitativa de bromidrato de fenoterol. A condição analítica empregada incluiu coluna em fase reversa C18 (150 mm × 3,9 mm d.i., 5 µm) Thermo®, fase móvel composta de mistura de acetonitrila e água (30:70, v/v) com 0,1% de trietilamina e pH ajustado para 5,0 com ácido fórmico, vazão de 1,0 mL.min-1 e detecção em UV a 276 nm. A faixa de linearidade foi de 0,025 a 0,15 mg.mL-1; a curva analítica mostrou coeficiente de correlação > 0,999. O limite de detecção (LD) foi de 0,003 mg.mL-1 e o limite de quantificação de 0,012 mg.mL-1. -

Future Diagnostic & Therapeutic Targets in Cardiorenal Syndromes
Future Diagnostic & Therapeutic Targets in Cardiorenal Syndromes (Biomarkers, advanced monitoring, advanced imaging, novel therapies) EDGAR V. LERMA, MD Clinical Professor of Medicine Secon of Nephrology UIC/ Advocate Christ Medical Center Oak Lawn, IL May 27, 2017 Disclosure of Interests • Honoraria: UpToDate, McGraw-Hill Publishing, Elsevier Publishing, Springer Publishing, Wolters-Kluwer Publishing, ACP Smart Medicine, Emedicine • Editorial Boards: American Journal of Kidney Diseases, ASN Kidney News, Clinical Journal of the American Society of Nephrology, Clinical Reviews in Bone and Mineral Metabolism, International Urology and Nephrology, Journal of Clinical Lipidology, Prescribers Letter, Renal and Urology News, Reviews in Endocrinology and Metabolic Disorders, Seminars in Dialysis • Speaker/ Advisory Board: Astute Medical, Mallinckrodt, Otsuka Pharmaceuticals, ZS Pharma KDIGO Controversies Conference on Heart Failure in CKD May 25-28, 2017 | Athens, Greece Disclosure of ABIM Service: Edgar V. Lerma, M.D. ▪ I am a current member of the ABIM Self-Assessment Committee. ▪ To protect the integrity of certification, ABIM enforces strict confidentiality and ownership of exam content. ▪ My participation in this CME activity is allowed under ABIM policy and is subject to the following: • As a member of an ABIM test committee, I agreed to keep exam information confidential, as it is owned exclusively by ABIM. • As is true for any ABIM candidate who has taken an exam for certification, I have signed the Pledge of Honesty in which I have agreed -

Ep 2560611 B1
(19) TZZ Z___T (11) EP 2 560 611 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) 03.01.2018 Bulletin 2018/01 (86) International application number: (21) Application number: 11719211.2 PCT/EP2011/056227 (22) Date of filing: 19.04.2011 (87) International publication number: WO 2011/131663 (27.10.2011 Gazette 2011/43) (54) "PROCESS FOR PROVIDING PARTICLES WITH REDUCED ELECTROSTATIC CHARGES" VERFAHREN ZUR BEREITSTELLUNG VON PARTIKELN MIT REDUZIERTEN ELEKTROSTATISCHEN LADUNGEN PROCÉDÉ DE PRÉPARATION DE PARTICULES AYANT DES CHARGES ÉLECTROSTATIQUES RÉDUITES (84) Designated Contracting States: • GUCHARDI ET AL: "Influence of fine lactose and AL AT BE BG CH CY CZ DE DK EE ES FI FR GB magnesium stearate on low dose dry powder GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO inhaler formulations", INTERNATIONAL PL PT RO RS SE SI SK SM TR JOURNAL OF PHARMACEUTICS, ELSEVIER BV, NL LNKD- DOI:10.1016/J.IJPHARM.2007.06.041, (30) Priority: 21.04.2010 EP 10160565 vol. 348, no. 1-2, 19 December 2007 (2007-12-19), pages 10-17, XP022393884, ISSN: 0378-5173 (43) Date of publication of application: • ELAJNAF A ET AL: "Electrostatic 27.02.2013 Bulletin 2013/09 characterisation of inhaled powders: Effect of contact surface and relative humidity", (73) Proprietor: Chiesi Farmaceutici S.p.A. EUROPEAN JOURNAL OF PHARMACEUTICAL 43100 Parma (IT) SCIENCES, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/J.EJPS.2006.07.006, vol. 29, no. 5, 1 (72) Inventors: December 2006 (2006-12-01), pages 375-384, • COCCONI, Daniela XP025137181, ISSN: 0928-0987 [retrieved on I-43100 Parma (IT) 2006-12-01] • MUSA, Rossella • CHAN ET AL: "Dry powder aerosol drug I-43100 Parma (IT) delivery-Opportunities for colloid and surface scientists", COLLOIDS AND SURFACES. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -
Ep 2626065 A1
(19) TZZ Z_T (11) EP 2 626 065 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: A61K 31/137 (2006.01) A61K 31/135 (2006.01) 14.08.2013 Bulletin 2013/33 A61K 31/4704 (2006.01) A61K 31/58 (2006.01) A61K 31/56 (2006.01) A61K 9/12 (2006.01) (2006.01) (2006.01) (21) Application number: 11827927.2 A61K 9/14 A61P 11/06 (86) International application number: Date of filing: 01.02.2011 (22) PCT/CN2011/070883 (87) International publication number: WO 2012/041031 (05.04.2012 Gazette 2012/14) (84) Designated Contracting States: (72) Inventor: WU, Wei-hsiu AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Taipei GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Taiwan (TW) PL PT RO RS SE SI SK SM TR (74) Representative: Patentanwaltskanzlei WILHELM (30) Priority: 28.09.2010 CN 201010502339 & BECK Prinzenstrasse 13 (71) Applicant: Intech Biopharm Ltd. 80639 München (DE) Taipei (TW) (54) COMPOUND COMPOSITION FOR INHALATION USED FOR TREATING ASTHMA (57) An inhaled pharmaceutical composition con- tric way as a controller. The eccentric way control therapy tains primary active ingredients of beta2- agonist and cor- could create a low blood concentration period during the ticosteroids.The pharmaceuticalcompositions disclosed day and minimize the acute tolerance phenomenon (or in the present invention are to be inhaled by a patient so called tachyphylaxis) for bronchodilator - beta2-ago- when needed as a reliever, or administrated in an eccen- nists in treating asthma or other obstructive respiratory disorders. -

5 August 2004 Version
The study listed may include approved and non-approved uses, formulations or treatment regimens. The results reported in any single study may not reflect the overall results obtained on studies of a product. Before prescribing any product mentioned in this Register, healthcare professionals should consult prescribing information for the product approved in their country. Study No.: SAM 30020 Title: Efficacy and tolerability of the salmeterol/fluticasone 50/100 mcg combination Diskus, inhaled once daily in the evening, in comparison to the p.r.n. inhalation from the reproterol/sodium cromoglycate 0.5/ 1 mg combination MDI, as initial therapy for patients with mild asthma – a multicentre, randomised, open, parallel trial Rationale: The reproterol/sodium cromoglycate 0.5/ 1 mg combination is still widely used for the treatment of mild asthmatics. Usually, patients inhale the combination product from an MDI, and they adjust the dosage according to their asthma symptoms. Anti-inflammatory medication with an inhaled corticosteroid such as fluticasone is an alternative therapeutic option, and long-acting beta agonists can be used as bronchodilatory treatment. The study aimed to investigate whether the efficacy of the salmeterol/fluticasone 50/100-mcg combination (SFC), inhaled from the Diskus once daily in the evening, is superior to that of the PRN inhalation of the reproterol/sodium cromoglycate 0.5/ 1 mg combination (RS) from the metered dose inhaler. Phase: IV Study Period: 13 June 2002 to 27 June 2003 Study Design: This randomised, prospective, open, parallel group study had a total duration of ten weeks. There were two study periods: a run-in period of two weeks, and a treatment period of 8 weeks. -

The Evolution of Heart Failure with Reduced Ejection Fraction Pharmacotherapy: What Do We Have and Where Are We Going?
Pharmacology & Therapeutics 178 (2017) 67–82 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate editor: M. Curtis The evolution of heart failure with reduced ejection fraction pharmacotherapy: What do we have and where are we going? Ahmed Selim, Ronald Zolty, Yiannis S. Chatzizisis ⁎ Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE, USA article info abstract Available online 21 March 2017 Cardiovascular diseases represent a leading cause of mortality and increased healthcare expenditure worldwide. Heart failure, which simply describes an inability of the heart to meet the body's needs, is the end point for many Keywords: other cardiovascular conditions. The last three decades have witnessed significant efforts aiming at the discovery Heart failure of treatments to improve the survival and quality of life of patients with heart failure; many were successful, Reduced ejection fraction while others failed. Given that most of the successes in treating heart failure were achieved in patients with re- Pharmacotherapy duced left ventricular ejection fraction (HFrEF), we constructed this review to look at the recent evolution of Novel drugs HFrEF pharmacotherapy. We also explore some of the ongoing clinical trials for new drugs, and investigate poten- tial treatment targets and pathways that might play a role in treating HFrEF in the future. © 2017 Elsevier Inc. All rights reserved. Contents 1. Introduction.............................................. -

Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin
fphar-07-00195 July 9, 2016 Time: 13:1 # 1 View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector ORIGINAL RESEARCH published: 12 July 2016 doi: 10.3389/fphar.2016.00195 Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin Veronika Wetzl1,2†, Elisabeth Schinner1†, Frieder Kees1, Franz Hofmann3, Lothar Faerber1,2 and Jens Schlossmann1* 1 Department of Pharmacology and Toxicology, University of Regensburg, Regensburg, Germany, 2 Novartis Pharma GmbH, Nuremberg, Germany, 3 Institute of Pharmacology and Toxicology, Technical University of Munich, Munich, Germany Introduction: Kidney fibrosis has shown to be ameliorated through the involvement of cyclic guanosine monophosphate (cGMP) and its dependent protein kinase I (cGKI). Serelaxin, the recombinant form of human relaxin-II, increases cGMP levels and has Edited by: Enno Klussmann, shown beneficial effects on kidney function in acute heart failure patients. Antifibrotic Max Delbrüeck Center for Molecular properties of serelaxin are supposed to be mediated via relaxin family peptide receptor Medicine, Germany 1 and subsequently enhanced nitric oxide/ cGMP to inhibit transforming growth factor- Reviewed by: Friedrich Wilhelm Herberg, b (TGF-b) signaling. This study examines the involvement of cGKI in the antifibrotic University of Kassel, Germany signaling of serelaxin. Choel Kim, Baylor College of Medicine, USA Methods and Results: Kidney fibrosis was induced by unilateral ureteral obstruction in *Correspondence: wildtype (WT) and cGKI knock-out (KO) mice. After 7 days, renal antifibrotic effects Jens Schlossmann of serelaxin were assessed. Serelaxin treatment for 7 days significantly increased [email protected] regensburg.de cGMP in the kidney of WT and cGKI-KO. -

Jimmunol.1800856.Full.Pdf
Healthy Donors Exhibit a CD4 T Cell Repertoire Specific to the Immunogenic Human Hormone H2-Relaxin before Injection This information is current as of September 26, 2021. Aurélien Azam, Yann Gallais, Sergio Mallart, Stephane Illiano, Olivier Duclos, Catherine Prades and Bernard Maillère J Immunol published online 17 May 2019 http://www.jimmunol.org/content/early/2019/05/14/jimmun Downloaded from ol.1800856 Supplementary http://www.jimmunol.org/content/suppl/2019/05/14/jimmunol.180085 http://www.jimmunol.org/ Material 6.DCSupplemental Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 26, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2019 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published May 17, 2019, doi:10.4049/jimmunol.1800856 The Journal of Immunology Healthy Donors Exhibit a CD4 T Cell Repertoire Specific to the Immunogenic Human Hormone H2-Relaxin before Injection Aure´lien Azam,*,† Yann Gallais,† Sergio Mallart,‡ Stephane Illiano,x Olivier Duclos,‡ Catherine Prades,* and Bernard Maille`re† H2-relaxin (RLN2) is a two-chain peptide hormone structurally related to insulin with a therapeutic potential in multiple indica- tions. -
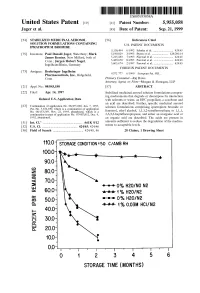
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int.