Reasanz; INN Serelaxin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Guidelines on Pulmonary Hypertension 2015 (TF08) - Task Force Members and Additional Contributors
Guidelines on Pulmonary Hypertension 2015 (TF08) - Task Force Members and Additional Contributors For ESC Guidelines: The report below lists declarations of interest as reported to the ESC by the experts covering the period of the Guidelines production, from Task Force creation to publication. Expert Type of Relationship with Industry Beghetti Maurice A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Novartis : Pulmonary hypertension imatinib (2012) - Pfizer : Pulmonary hypertension sildenafil (2012-2013) - Bayer Schering Pharma : Pulmonary hypertension riociguat (2012-2013-2014-2015) - Eli Lilly : Pulmonary hypertension tadalafil (2012-2013-2014-2015) - Actelion : Pulmonary hypertension, Tracleer, Macitentan, Selexipag (2012-2013-2014-2015) - GlaxoSmithKline : pulmonary hypertension Ambrisentan (2012-2015) - Novartis : Pulmonary hypertension riociguat (2013) - GlaxoSmithKline : ambrisentan (2014) D - Research funding (departmental or institutional). - Actelion : no relation to a specific product (2012-2013) - Bayer Schering Pharma : no relation to a specific product (2014-2015) Galie Nazzareno A - Direct Personal payment: Speaker fees, Honoraria, Consultancy, Advisory Board fees, Investigator, Committee Member, etc. - Eli Lilly : pulmonary hypertension (2012-2013) - Novartis : pulmonary hypertension (2012-2013) - Pfizer : pulmonary hypertension (2012-2013) - Actelion : pulmonary hypertension (2012-2013) - GlaxoSmithKline : pulmonary hypertension (2012-2013) -

Future Diagnostic & Therapeutic Targets in Cardiorenal Syndromes
Future Diagnostic & Therapeutic Targets in Cardiorenal Syndromes (Biomarkers, advanced monitoring, advanced imaging, novel therapies) EDGAR V. LERMA, MD Clinical Professor of Medicine Secon of Nephrology UIC/ Advocate Christ Medical Center Oak Lawn, IL May 27, 2017 Disclosure of Interests • Honoraria: UpToDate, McGraw-Hill Publishing, Elsevier Publishing, Springer Publishing, Wolters-Kluwer Publishing, ACP Smart Medicine, Emedicine • Editorial Boards: American Journal of Kidney Diseases, ASN Kidney News, Clinical Journal of the American Society of Nephrology, Clinical Reviews in Bone and Mineral Metabolism, International Urology and Nephrology, Journal of Clinical Lipidology, Prescribers Letter, Renal and Urology News, Reviews in Endocrinology and Metabolic Disorders, Seminars in Dialysis • Speaker/ Advisory Board: Astute Medical, Mallinckrodt, Otsuka Pharmaceuticals, ZS Pharma KDIGO Controversies Conference on Heart Failure in CKD May 25-28, 2017 | Athens, Greece Disclosure of ABIM Service: Edgar V. Lerma, M.D. ▪ I am a current member of the ABIM Self-Assessment Committee. ▪ To protect the integrity of certification, ABIM enforces strict confidentiality and ownership of exam content. ▪ My participation in this CME activity is allowed under ABIM policy and is subject to the following: • As a member of an ABIM test committee, I agreed to keep exam information confidential, as it is owned exclusively by ABIM. • As is true for any ABIM candidate who has taken an exam for certification, I have signed the Pledge of Honesty in which I have agreed -

Drug Class Review Antianginal Agents
Drug Class Review Antianginal Agents 24:12.08 Nitrates and Nitrites 24:04.92 Cardiac Drugs, Miscellaneous Amyl Nitrite Isosorbide Dinitrate (IsoDitrate ER®, others) Isosorbide Mononitrate (Imdur®) Nitroglycerin (Minitran®, Nitrostat®, others) Ranolazine (Ranexa®) Final Report May 2015 Review prepared by: Melissa Archer, PharmD, Clinical Pharmacist Carin Steinvoort, PharmD, Clinical Pharmacist Gary Oderda, PharmD, MPH, Professor University of Utah College of Pharmacy Copyright © 2015 by University of Utah College of Pharmacy Salt Lake City, Utah. All rights reserved. Table of Contents Executive Summary ......................................................................................................................... 3 Introduction .................................................................................................................................... 4 Table 1. Antianginal Therapies .............................................................................................. 4 Table 2. Summary of Agents .................................................................................................. 5 Disease Overview ........................................................................................................................ 8 Table 3. Summary of Current Clinical Practice Guidelines .................................................... 9 Pharmacology ............................................................................................................................... 10 Table 4. Pharmacokinetic Properties -

List of Union Reference Dates A
Active substance name (INN) EU DLP BfArM / BAH DLP yearly PSUR 6-month-PSUR yearly PSUR bis DLP (List of Union PSUR Submission Reference Dates and Frequency (List of Union Frequency of Reference Dates and submission of Periodic Frequency of submission of Safety Update Reports, Periodic Safety Update 30 Nov. 2012) Reports, 30 Nov. -

Classification Decisions Taken by the Harmonized System Committee from the 47Th to 60Th Sessions (2011
CLASSIFICATION DECISIONS TAKEN BY THE HARMONIZED SYSTEM COMMITTEE FROM THE 47TH TO 60TH SESSIONS (2011 - 2018) WORLD CUSTOMS ORGANIZATION Rue du Marché 30 B-1210 Brussels Belgium November 2011 Copyright © 2011 World Customs Organization. All rights reserved. Requests and inquiries concerning translation, reproduction and adaptation rights should be addressed to [email protected]. D/2011/0448/25 The following list contains the classification decisions (other than those subject to a reservation) taken by the Harmonized System Committee ( 47th Session – March 2011) on specific products, together with their related Harmonized System code numbers and, in certain cases, the classification rationale. Advice Parties seeking to import or export merchandise covered by a decision are advised to verify the implementation of the decision by the importing or exporting country, as the case may be. HS codes Classification No Product description Classification considered rationale 1. Preparation, in the form of a powder, consisting of 92 % sugar, 6 % 2106.90 GRIs 1 and 6 black currant powder, anticaking agent, citric acid and black currant flavouring, put up for retail sale in 32-gram sachets, intended to be consumed as a beverage after mixing with hot water. 2. Vanutide cridificar (INN List 100). 3002.20 3. Certain INN products. Chapters 28, 29 (See “INN List 101” at the end of this publication.) and 30 4. Certain INN products. Chapters 13, 29 (See “INN List 102” at the end of this publication.) and 30 5. Certain INN products. Chapters 28, 29, (See “INN List 103” at the end of this publication.) 30, 35 and 39 6. Re-classification of INN products. -

Heart Failure
9/15/2010 HEART FAILURE PfProfessor LlLexley M MPitP Pinto Perei ra Pharmacology Unit Faculty of Medical Sciences UWI ST Augustine Acknowledgements : American Heart Association Sociedad Española de CardiologíaJune, 1999 Epidemiology • More in N Europe and USA • Increases with age esp in males > 75 • US Data • 5,000,000 patients • 6,500,000 hospital days/ year • 300,000 deaths / year • 6% --10%10% of people > 65 years • 5.4%f% of health care budget (38 billion) • Incidence x 2 in last ten years • Estimated prevalence 6 mio by 2030 Gottdiener J et al. JACC 2000;35:1628, Haldeman GA et al. Am Heart J 1999;137:352 Kannel WB et al. Am Heart J 1991;121:951, O’Connell JB et al. J Heart Lung Transplant 1993;13:S107, Young J, MCNA 2004; 1 9/15/2010 Definition of heart failure Clinical syyyndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill with or eject blood AHA / ACC HF guidelines 2001 Clinical symptoms / signs secondary to abnormal ventricular function ESC HF guidelines 2001 “Heart Failure” vs. “Congestive Heart Failure” All patients may NOT have volume overload at the time of initial or subsequent evaluation. Therefore the term “heart failure” is preferred over the older term “ihfil”“congestive heart failure.” CHF is a significant cause of morbidity and mortality Important cause of hospitalizations among elderly 2 9/15/2010 Causes of HF For a substantial proportion of patients, the causes of HF are: 1. Coronary artery disease 2. Hypertension 3. Dilated cardiomyopathy And there are other causes 1. -

The Evolution of Heart Failure with Reduced Ejection Fraction Pharmacotherapy: What Do We Have and Where Are We Going?
Pharmacology & Therapeutics 178 (2017) 67–82 Contents lists available at ScienceDirect Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/pharmthera Associate editor: M. Curtis The evolution of heart failure with reduced ejection fraction pharmacotherapy: What do we have and where are we going? Ahmed Selim, Ronald Zolty, Yiannis S. Chatzizisis ⁎ Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE, USA article info abstract Available online 21 March 2017 Cardiovascular diseases represent a leading cause of mortality and increased healthcare expenditure worldwide. Heart failure, which simply describes an inability of the heart to meet the body's needs, is the end point for many Keywords: other cardiovascular conditions. The last three decades have witnessed significant efforts aiming at the discovery Heart failure of treatments to improve the survival and quality of life of patients with heart failure; many were successful, Reduced ejection fraction while others failed. Given that most of the successes in treating heart failure were achieved in patients with re- Pharmacotherapy duced left ventricular ejection fraction (HFrEF), we constructed this review to look at the recent evolution of Novel drugs HFrEF pharmacotherapy. We also explore some of the ongoing clinical trials for new drugs, and investigate poten- tial treatment targets and pathways that might play a role in treating HFrEF in the future. © 2017 Elsevier Inc. All rights reserved. Contents 1. Introduction.............................................. -

Effects of Intravenous Nicorandil on the Mid-Term Prognosis of Patients
Circulation Journal ORIGINAL ARTICLE Official Journal of the Japanese Circulation Society http://www.j-circ.or.jp Heart Failure Effects of Intravenous Nicorandil on the Mid-Term Prognosis of Patients With Acute Heart Failure Syndrome Shiro Ishihara, MD; Tokushi Koga, MD; Shigeru Kaseda, MD; Eiji Nyuta, MD; Yoshie Haga, MD; Shinichiro Fujishima, MD; Takao Ishitsuka, MD; Seizo Sadoshima, MD Background: Acute heart failure syndrome (AHFS) remains a major clinical challenge because of its poor prognosis. Nicorandil, a hybrid compound of a potassium-channel opener and nitric oxide donor, has been reported to improve the prognosis of ischemic heart disease. We sought to evaluate the effect of intravenous nicorandil on the mid-term prognosis of AHFS. Methods and Results: A total of 402 consecutive patients who were hospitalized for AHFS were divided into 2 groups according to the use of intravenous nicorandil: 78 patients in the Nicorandil group and 324 patients in the Control group. During the 180-day follow-up, death or rehospitalization for heart failure occurred in 7 patients in the Nicorandil group (9.0%) and in 75 patients (23.2%) in the Control group. Event-free survival rates were significantly higher in the Nicorandil group than in the Control group (P=0.006). Multivariate Cox hazard analysis revealed that age (hazard ratio (HR)=1.066, P<0.0001), systolic blood pressure (HR=0.983, P=0.0023), New York Heart Association class III/IV (HR=6.550, P<0.0001), log creatinine (HR=3.866, P=0.0106), and use of intravenous nicorandil (HR=0.179, P<0.0001) were significant predictive factors for the occurrence of death or rehospitalization for heart failure. -

Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin
fphar-07-00195 July 9, 2016 Time: 13:1 # 1 View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Frontiers - Publisher Connector ORIGINAL RESEARCH published: 12 July 2016 doi: 10.3389/fphar.2016.00195 Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin Veronika Wetzl1,2†, Elisabeth Schinner1†, Frieder Kees1, Franz Hofmann3, Lothar Faerber1,2 and Jens Schlossmann1* 1 Department of Pharmacology and Toxicology, University of Regensburg, Regensburg, Germany, 2 Novartis Pharma GmbH, Nuremberg, Germany, 3 Institute of Pharmacology and Toxicology, Technical University of Munich, Munich, Germany Introduction: Kidney fibrosis has shown to be ameliorated through the involvement of cyclic guanosine monophosphate (cGMP) and its dependent protein kinase I (cGKI). Serelaxin, the recombinant form of human relaxin-II, increases cGMP levels and has Edited by: Enno Klussmann, shown beneficial effects on kidney function in acute heart failure patients. Antifibrotic Max Delbrüeck Center for Molecular properties of serelaxin are supposed to be mediated via relaxin family peptide receptor Medicine, Germany 1 and subsequently enhanced nitric oxide/ cGMP to inhibit transforming growth factor- Reviewed by: Friedrich Wilhelm Herberg, b (TGF-b) signaling. This study examines the involvement of cGKI in the antifibrotic University of Kassel, Germany signaling of serelaxin. Choel Kim, Baylor College of Medicine, USA Methods and Results: Kidney fibrosis was induced by unilateral ureteral obstruction in *Correspondence: wildtype (WT) and cGKI knock-out (KO) mice. After 7 days, renal antifibrotic effects Jens Schlossmann of serelaxin were assessed. Serelaxin treatment for 7 days significantly increased [email protected] regensburg.de cGMP in the kidney of WT and cGKI-KO. -

Genetic Factors Influencing B-Type Natriuretic Peptide-Mediated Production of Cyclic Guanosine Monophosphate and Blood Pressure Effects in Heart Failure Patients
Henry Ford Health System Henry Ford Health System Scholarly Commons Cardiology Articles Cardiology/Cardiovascular Research 12-1-2015 Genetic Factors Influencing B-type Natriuretic Peptide-Mediated Production of Cyclic Guanosine Monophosphate and Blood Pressure Effects in Heart Failure Patients David E. Lanfear Henry Ford Health System, [email protected] Jia Li Henry Ford Health System, [email protected] Raza Abbas Ruicong She Henry Ford Health System, [email protected] Badri Padhukasahasram See next page for additional authors Follow this and additional works at: https://scholarlycommons.henryford.com/cardiology_articles Recommended Citation Lanfear DE, Li J, Abbas R, She R, Padhukasahasram B, Gupta RC, Langholz D, Tang WH, Williams LK, Sabbah HN, Chow SL. Genetic factors influencing b-type natriuretic peptide-mediated production of cyclic guanosine monophosphate and blood pressure effects in heart failure patients. J Cardiovasc Transl Res. 2015 ;8(9):545-53. This Article is brought to you for free and open access by the Cardiology/Cardiovascular Research at Henry Ford Health System Scholarly Commons. It has been accepted for inclusion in Cardiology Articles by an authorized administrator of Henry Ford Health System Scholarly Commons. Authors David E. Lanfear, Jia Li, Raza Abbas, Ruicong She, Badri Padhukasahasram, Ramesh C. Gupta, David Langholz, W HW Tang, Keoki L. Williams, Hani N. Sabbah, and Sheryl L. Chow This article is available at Henry Ford Health System Scholarly Commons: https://scholarlycommons.henryford.com/ cardiology_articles/409 J. of Cardiovasc. Trans. Res. (2015) 8:545–553 DOI 10.1007/s12265-015-9660-2 Genetic Factors Influencing B-type Natriuretic Peptide-Mediated Production of Cyclic Guanosine Monophosphate and Blood Pressure Effects in Heart Failure Patients David E. -

Jimmunol.1800856.Full.Pdf
Healthy Donors Exhibit a CD4 T Cell Repertoire Specific to the Immunogenic Human Hormone H2-Relaxin before Injection This information is current as of September 26, 2021. Aurélien Azam, Yann Gallais, Sergio Mallart, Stephane Illiano, Olivier Duclos, Catherine Prades and Bernard Maillère J Immunol published online 17 May 2019 http://www.jimmunol.org/content/early/2019/05/14/jimmun Downloaded from ol.1800856 Supplementary http://www.jimmunol.org/content/suppl/2019/05/14/jimmunol.180085 http://www.jimmunol.org/ Material 6.DCSupplemental Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 26, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2019 by The American Association of Immunologists, Inc. All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. Published May 17, 2019, doi:10.4049/jimmunol.1800856 The Journal of Immunology Healthy Donors Exhibit a CD4 T Cell Repertoire Specific to the Immunogenic Human Hormone H2-Relaxin before Injection Aure´lien Azam,*,† Yann Gallais,† Sergio Mallart,‡ Stephane Illiano,x Olivier Duclos,‡ Catherine Prades,* and Bernard Maille`re† H2-relaxin (RLN2) is a two-chain peptide hormone structurally related to insulin with a therapeutic potential in multiple indica- tions. -
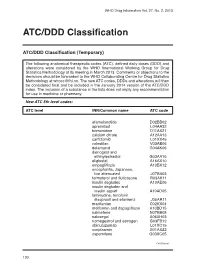
ATC/DDD Classification
WHO Drug Information Vol. 27, No. 2, 2013 ATC/DDD Classification ATC/DDD Classification (Temporary) The following anatomical therapeutic codes (ATC), defined daily doses (DDD) and alterations were considered by the WHO International Working Group for Drug Statistics Methodology at its meeting in March 2013. Comments or objections to the decisions should be forwarded to the WHO Collaborating Centre for Drug Statistics Methodology at [email protected]. The new ATC codes, DDDs and alterations will then be considered final and be included in the January 2014 version of the ATC/DDD index. The inclusion of a substance in the lists does not imply any recommendation for use in medicine or pharmacy. New ATC 5th level codes: ATC level INN/Common name ATC code afamelanotide D02BB02 apremilast L04AA32 brimonidine D11AX21 calcium citrate A12AA13 carfilzomib L01XX45 colestilan V03AE06 delamanid G04AK06 dienogest and ethinylestradiol G03AA16 eliglustat A16AX10 empagliflozin A10BX12 encephalitis, Japanese, live attenuated J07BA03 formoterol and fluticasone R03AK11 insulin degludec A10AE06 insulin degludec and insulin aspart A10AD05 lamivudine, tenofovir disoproxil and efavirenz J05AR11 macitentan C02KX04 metformin and dapagliflozin A10BD15 nalmefene N07BB05 naloxegol A06AH03 nomegestrol and estrogen G03FB12 obinutuzumab L01XC15 ocriplasmin S01AX22 ospemifene G03XC05 Continued/ 130 WHO Drug Information Vol. 27, No. 2, 2013 ATC/DDD Classification ATC level INN/Common name ATC code pomalidomide L04AX06 serelaxin C01DX21 strontium ranelate and colecalciferol