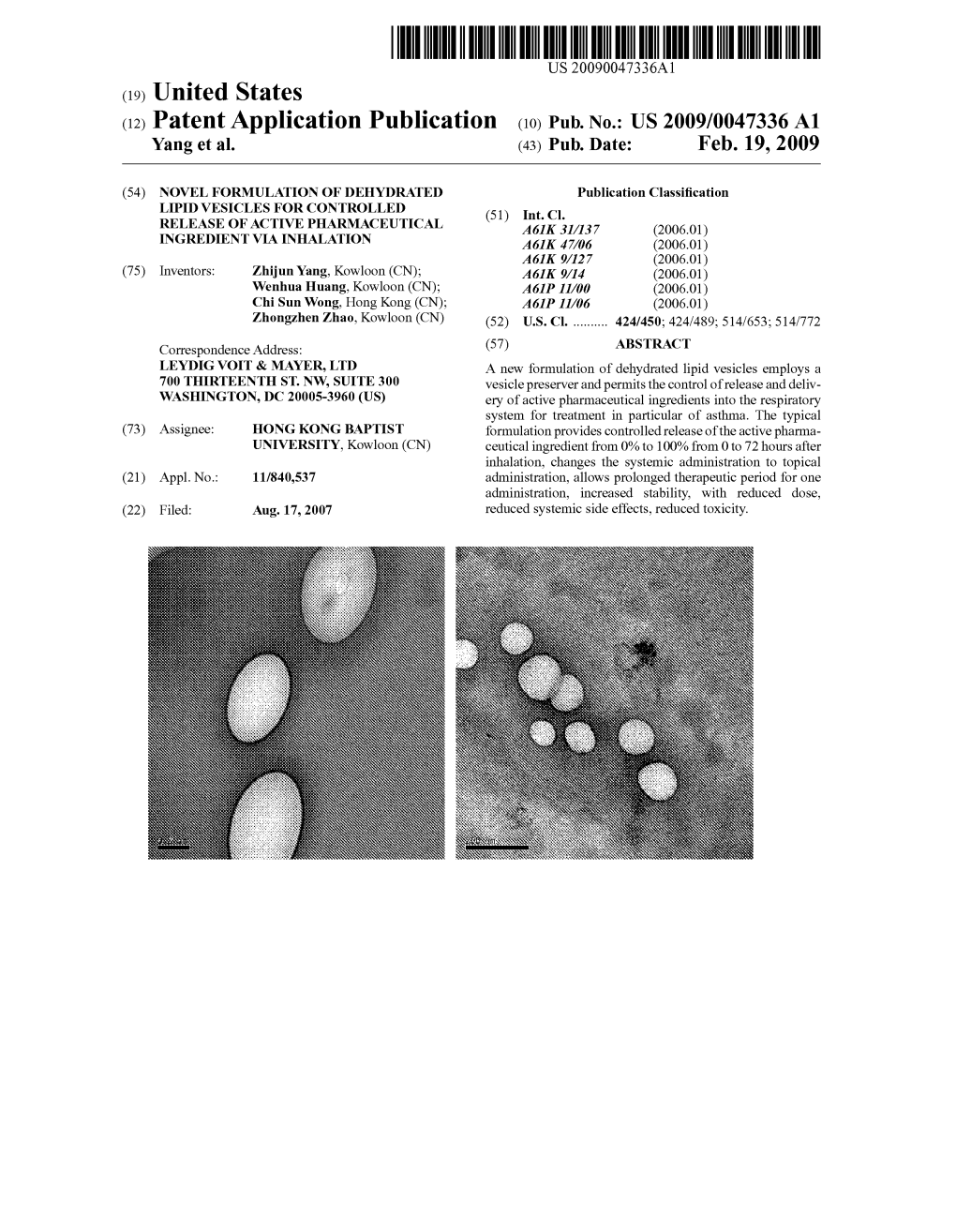
(12) Patent Application Publication (10) Pub. No.: US 2009/0047336A1 Yang Et Al
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Validation of New Analytical Methodology for Determining Fenoterolhydrobromide by HPLC: Application in Pharmaceutical Products
Artigo Original/Original Article Validation of new analytical methodology for determining fenoterolhydrobromide by HPLC: application in pharmaceutical products Validação de uma nova metodologia analítica para determinação de bromidrato de fenoterol por CLAE: aplicações em produtos farmacêuticos RIALA6/1475 *Helena Miyoco YANO1, Fernanda Fernandes FARIAS1, Marcelo Beiriz DEL BIANCO1, Pedro Lopez GARCIA2 *Endereço para correspondência: 1Núcleo de Ensaios Físicos e Químicos em Medicamentos, Centro de Medicamentos, Cosméticos e Saneantes, Instituto Adolfo Lutz, Av. Doutor Arnaldo, 355, CEP: 01246-902, São Paulo, SP, Brasil. E-mail: [email protected] 2Departamento de Farmácia, Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, São Paulo, SP, Brasil. Recebido: 31.10.2011- Aceito para publicação: 20.04.2012 RESUMO Bromidrato de fenoterol é um agente agonista adrenérgico β2-seletivo utilizado para tratamento de asma e doenças pulmonares crônicas obstrutivas. A metodologia analítica por cromatografia líquida de alta eficiência (CLAE) foi desenvolvida e validada para a determinação quantitativa de bromidrato de fenoterol. A condição analítica empregada incluiu coluna em fase reversa C18 (150 mm × 3,9 mm d.i., 5 µm) Thermo®, fase móvel composta de mistura de acetonitrila e água (30:70, v/v) com 0,1% de trietilamina e pH ajustado para 5,0 com ácido fórmico, vazão de 1,0 mL.min-1 e detecção em UV a 276 nm. A faixa de linearidade foi de 0,025 a 0,15 mg.mL-1; a curva analítica mostrou coeficiente de correlação > 0,999. O limite de detecção (LD) foi de 0,003 mg.mL-1 e o limite de quantificação de 0,012 mg.mL-1. -

Classification of Medicinal Drugs and Driving: Co-Ordination and Synthesis Report
Project No. TREN-05-FP6TR-S07.61320-518404-DRUID DRUID Driving under the Influence of Drugs, Alcohol and Medicines Integrated Project 1.6. Sustainable Development, Global Change and Ecosystem 1.6.2: Sustainable Surface Transport 6th Framework Programme Deliverable 4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Due date of deliverable: 21.07.2011 Actual submission date: 21.07.2011 Revision date: 21.07.2011 Start date of project: 15.10.2006 Duration: 48 months Organisation name of lead contractor for this deliverable: UVA Revision 0.0 Project co-funded by the European Commission within the Sixth Framework Programme (2002-2006) Dissemination Level PU Public PP Restricted to other programme participants (including the Commission x Services) RE Restricted to a group specified by the consortium (including the Commission Services) CO Confidential, only for members of the consortium (including the Commission Services) DRUID 6th Framework Programme Deliverable D.4.4.1 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Page 1 of 243 Classification of medicinal drugs and driving: Co-ordination and synthesis report. Authors Trinidad Gómez-Talegón, Inmaculada Fierro, M. Carmen Del Río, F. Javier Álvarez (UVa, University of Valladolid, Spain) Partners - Silvia Ravera, Susana Monteiro, Han de Gier (RUGPha, University of Groningen, the Netherlands) - Gertrude Van der Linden, Sara-Ann Legrand, Kristof Pil, Alain Verstraete (UGent, Ghent University, Belgium) - Michel Mallaret, Charles Mercier-Guyon, Isabelle Mercier-Guyon (UGren, University of Grenoble, Centre Regional de Pharmacovigilance, France) - Katerina Touliou (CERT-HIT, Centre for Research and Technology Hellas, Greece) - Michael Hei βing (BASt, Bundesanstalt für Straßenwesen, Germany). -

Ep 2560611 B1
(19) TZZ Z___T (11) EP 2 560 611 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) 03.01.2018 Bulletin 2018/01 (86) International application number: (21) Application number: 11719211.2 PCT/EP2011/056227 (22) Date of filing: 19.04.2011 (87) International publication number: WO 2011/131663 (27.10.2011 Gazette 2011/43) (54) "PROCESS FOR PROVIDING PARTICLES WITH REDUCED ELECTROSTATIC CHARGES" VERFAHREN ZUR BEREITSTELLUNG VON PARTIKELN MIT REDUZIERTEN ELEKTROSTATISCHEN LADUNGEN PROCÉDÉ DE PRÉPARATION DE PARTICULES AYANT DES CHARGES ÉLECTROSTATIQUES RÉDUITES (84) Designated Contracting States: • GUCHARDI ET AL: "Influence of fine lactose and AL AT BE BG CH CY CZ DE DK EE ES FI FR GB magnesium stearate on low dose dry powder GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO inhaler formulations", INTERNATIONAL PL PT RO RS SE SI SK SM TR JOURNAL OF PHARMACEUTICS, ELSEVIER BV, NL LNKD- DOI:10.1016/J.IJPHARM.2007.06.041, (30) Priority: 21.04.2010 EP 10160565 vol. 348, no. 1-2, 19 December 2007 (2007-12-19), pages 10-17, XP022393884, ISSN: 0378-5173 (43) Date of publication of application: • ELAJNAF A ET AL: "Electrostatic 27.02.2013 Bulletin 2013/09 characterisation of inhaled powders: Effect of contact surface and relative humidity", (73) Proprietor: Chiesi Farmaceutici S.p.A. EUROPEAN JOURNAL OF PHARMACEUTICAL 43100 Parma (IT) SCIENCES, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/J.EJPS.2006.07.006, vol. 29, no. 5, 1 (72) Inventors: December 2006 (2006-12-01), pages 375-384, • COCCONI, Daniela XP025137181, ISSN: 0928-0987 [retrieved on I-43100 Parma (IT) 2006-12-01] • MUSA, Rossella • CHAN ET AL: "Dry powder aerosol drug I-43100 Parma (IT) delivery-Opportunities for colloid and surface scientists", COLLOIDS AND SURFACES. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Genl:VE 1970 © World Health Organization 1970
Nathan B. Eddy, Hans Friebel, Klaus-Jiirgen Hahn & Hans Halbach WORLD HEALTH ORGANIZATION ORGANISATION .MONDIALE DE LA SANT~ GENl:VE 1970 © World Health Organization 1970 Publications of the World Health Organization enjoy copyright protection in accordance with the provisions of Protocol 2 of the Universal Copyright Convention. Nevertheless governmental agencies or learned and professional societies may reproduce data or excerpts or illustrations from them without requesting an authorization from the World Health Organization. For rights of reproduction or translation of WHO publications in toto, application should be made to the Division of Editorial and Reference Services, World Health Organization, Geneva, Switzerland. The World Health Organization welcomes such applications. Authors alone are responsible for views expressed in signed articles. The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the Director-General of the World Health Organization concerning the legal status of any country or territory or of its authorities, or concerning the delimitation of its frontiers. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. © Organisation mondiale de la Sante 1970 Les publications de l'Organisation mondiale de la Sante beneficient de la protection prevue par les dispositions du Protocole n° 2 de la Convention universelle pour la Protection du Droit d'Auteur. Les institutions gouvernementales et les societes savantes ou professionnelles peuvent, toutefois, reproduire des donnees, des extraits ou des illustrations provenant de ces publications, sans en demander l'autorisation a l'Organisation mondiale de la Sante. Pour toute reproduction ou traduction integrate, une autorisation doit etre demandee a la Division des Services d'Edition et de Documentation, Organisation mondiale de la Sante, Geneve, Suisse. -

ADD/ADHD: Strattera • Allergy/Anti-Inflammatories
EXAMPLES OF PERMITTED MEDICATIONS - 2015 ADD/ADHD: Strattera Allergy/Anti-Inflammatories: Corticosteroids, including Decadron, Depo-Medrol, Entocort, Solu-Medrol, Prednisone, Prednisolone, and Methylprednisolone Anesthetics: Alcaine, Articadent, Bupivacaine HCI, Chloroprocaine, Citanest Plain Dental, Itch-X, Lidocaine, Marcaine, Mepivacaine HCI, Naropin, Nesacaine, Novacain, Ophthetic, Oraqix, Paracaine, Polocaine, Pontocaine Hydrochloride, PrameGel, Prax, Proparacaine HCI, Ropivacaine, Sarna Ultra, Sensorcaine, Synera, Tetracaine, Tronothane HCI, and Xylocaine Antacids: Calci-Chew, Di-Gel, Gaviscon, Gelusil, Maalox, Mintox Plus, Mylanta, Oyst-Cal 500, Rolaids, and Tums Anti-Anxiety: Alprazolam, Atarax, Ativan, Buspar, Buspirone HCI, Chlordiazepoxide HCI, Clonazepam, Chlorazepate Dipotassium, Diastat, Diazepam, Hydroxyzine, Klonopin, Librium, Lorazepam, Niravam, Tranxene T-tab, Valium, Vistaril, and Xanax Antibiotics: Acetasol HC, Amoxil, Ampicillin, Antiben, Antibiotic-Cort, Antihist, Antituss, Avelox, Ceftazidime, Ceftin, Cefuroxime Axetil, Ceptaz, Cleocin, Cloxapen, Cortane-B Aqueous, Cortic, Cresylate, Debrox, Doryx, EarSol-HC, Fortaz, Gantrisin, Mezlin, Moxifloxacin, Neotic, Otocain, Principen, Tazicef, Tazidime, Trioxin, and Zyvox Anti-Depressants: Adapin, Anafranil, Asendin, Bolvidon, Celexa, Cymbalata, Deprilept, Effexor, Elavil, Lexapro, Luvox, Norpramin, Pamelor, Paxil, Pristiq, Prozac, Savella, Surmontil, Tofranil, Vivactil, Wellbutrin, Zoloft, and Zyban Anti-Diabetics: Actos, Amaryl, Avandia, Glipizide, Glucophage, -

Title 16. Crimes and Offenses Chapter 13. Controlled Substances Article 1
TITLE 16. CRIMES AND OFFENSES CHAPTER 13. CONTROLLED SUBSTANCES ARTICLE 1. GENERAL PROVISIONS § 16-13-1. Drug related objects (a) As used in this Code section, the term: (1) "Controlled substance" shall have the same meaning as defined in Article 2 of this chapter, relating to controlled substances. For the purposes of this Code section, the term "controlled substance" shall include marijuana as defined by paragraph (16) of Code Section 16-13-21. (2) "Dangerous drug" shall have the same meaning as defined in Article 3 of this chapter, relating to dangerous drugs. (3) "Drug related object" means any machine, instrument, tool, equipment, contrivance, or device which an average person would reasonably conclude is intended to be used for one or more of the following purposes: (A) To introduce into the human body any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (B) To enhance the effect on the human body of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; (C) To conceal any quantity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state; or (D) To test the strength, effectiveness, or purity of any dangerous drug or controlled substance under circumstances in violation of the laws of this state. (4) "Knowingly" means having general knowledge that a machine, instrument, tool, item of equipment, contrivance, or device is a drug related object or having reasonable grounds to believe that any such object is or may, to an average person, appear to be a drug related object. -

Pharmacology on Your Palms CLASSIFICATION of the DRUGS
Pharmacology on your palms CLASSIFICATION OF THE DRUGS DRUGS FROM DRUGS AFFECTING THE ORGANS CHEMOTHERAPEUTIC DIFFERENT DRUGS AFFECTING THE NERVOUS SYSTEM AND TISSUES DRUGS PHARMACOLOGICAL GROUPS Drugs affecting peripheral Antitumor drugs Drugs affecting the cardiovascular Antimicrobial, antiviral, Drugs affecting the nervous system Antiallergic drugs system antiparasitic drugs central nervous system Drugs affecting the sensory Antidotes nerve endings Cardiac glycosides Antibiotics CNS DEPRESSANTS (AFFECTING THE Antihypertensive drugs Sulfonamides Analgesics (opioid, AFFERENT INNERVATION) Antianginal drugs Antituberculous drugs analgesics-antipyretics, Antiarrhythmic drugs Antihelminthic drugs NSAIDs) Local anaesthetics Antihyperlipidemic drugs Antifungal drugs Sedative and hypnotic Coating drugs Spasmolytics Antiviral drugs drugs Adsorbents Drugs affecting the excretory system Antimalarial drugs Tranquilizers Astringents Diuretics Antisyphilitic drugs Neuroleptics Expectorants Drugs affecting the hemopoietic system Antiseptics Anticonvulsants Irritant drugs Drugs affecting blood coagulation Disinfectants Antiparkinsonian drugs Drugs affecting peripheral Drugs affecting erythro- and leukopoiesis General anaesthetics neurotransmitter processes Drugs affecting the digestive system CNS STIMULANTS (AFFECTING THE Anorectic drugs Psychomotor stimulants EFFERENT PART OF THE Bitter stuffs. Drugs for replacement therapy Analeptics NERVOUS SYSTEM) Antiacid drugs Antidepressants Direct-acting-cholinomimetics Antiulcer drugs Nootropics (Cognitive -

UNIVERSITE DE NANTES Thomas Gelineau
UNIVERSITE DE NANTES __________ FACULTE DE MEDECINE __________ Année 2011 N° 139 THESE pour le DIPLÔME D’ÉTAT DE DOCTEUR EN MÉDECINE DES de médecine générale par Thomas Gelineau né le 29 janvier 1983 à Cholet __________ Présentée et soutenue publiquement le 06/12/2011 __________ LE RHUME DE L'ENFANT ET SON TRAITEMENT: DECISION PARTAGEE AVEC LES PARENTS D'APRES UN QUESTIONNAIRE __________ Président : Monsieur le Professeur Olivier MALARD Directeur de thèse : Madame le Professeur Jacqueline LACAILLE 1 Table des matières IIntroduction................................................................................................................ 7 IIDéfinition, état des connaissances...........................................................................8 1Le rhume.......................................................................................................................................8 APhysiopathologie............................................................................................................................. 8 aL'origine virale.................................................................................................................................... 8 bLa saisonnalité................................................................................................................................... 8 cL'âge de survenue.............................................................................................................................. 9 dLe sexe.................................................................................................................................................. -

Partial Agreement in the Social and Public Health Field
COUNCIL OF EUROPE COMMITTEE OF MINISTERS (PARTIAL AGREEMENT IN THE SOCIAL AND PUBLIC HEALTH FIELD) RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies, and superseding Resolution AP (82) 2) AND APPENDIX I Alphabetical list of medicines adopted by the Public Health Committee (Partial Agreement) updated to 1 July 1988 APPENDIX II Pharmaco-therapeutic classification of medicines appearing in the alphabetical list in Appendix I updated to 1 July 1988 RESOLUTION AP (88) 2 ON THE CLASSIFICATION OF MEDICINES WHICH ARE OBTAINABLE ONLY ON MEDICAL PRESCRIPTION (superseding Resolution AP (82) 2) (Adopted by the Committee of Ministers on 22 September 1988 at the 419th meeting of the Ministers' Deputies) The Representatives on the Committee of Ministers of Belgium, France, the Federal Republic of Germany, Italy, Luxembourg, the Netherlands and the United Kingdom of Great Britain and Northern Ireland, these states being parties to the Partial Agreement in the social and public health field, and the Representatives of Austria, Denmark, Ireland, Spain and Switzerland, states which have participated in the public health activities carried out within the above-mentioned Partial Agreement since 1 October 1974, 2 April 1968, 23 September 1969, 21 April 1988 and 5 May 1964, respectively, Considering that the aim of the Council of Europe is to achieve greater unity between its members and that this -
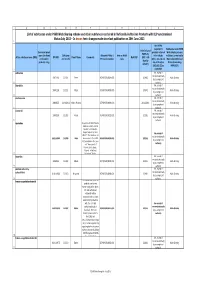
List of Substances Under PSUR WS Scheme and Other Substances
ABCDEFGHIJKL List of substances under PSUR Work Sharing scheme and other substances contained in Nationally Authorised Products with DLP synchronised Status July 2013 In brown font: changes made since last publication on 30th June 2013 1 Are PSURs required for Substances under PSUR Submission of Innovator brand products referred Work Sharing scheme PSURs by name (for fixed DLP (year Allocated PRMS / Note on PSUR to in Articles or Others (contained in Active substance name (INN) EU HBD Firm's Name Comments Next DLP (DLP + 90 combination and month) Procedure number cycle 10(1), 10a, 14 and Nationally Authorised days by products only) 16a of Directive Products including default) 2001/83/EC as MRP/DCP) 2 amended? sulbactam No, except if required nationally 19871116 201311 Pfizer AT/H/PSUR/0004/003 201402 Work Sharing by a competent 3 authority idarubicin No, except if required nationally 19891129 201311 Pfizer AT/H/PSUR/0005/003 201402 Work Sharing by a competent 4 authority dexibuprofen No, except if required nationally 20000725 31/08/2014 Gebro Pharma AT/H/PSUR/0009/003 29/11/2014 Work Sharing by a competent 5 authority nicorandil No, except if required nationally 19900209 201302 Merck AT/H/PSUR/0023/002 201305 Work Sharing by a competent 6 authority azelastine Request from MEDA Pharma GmbH & Co.KG to amend the DLP to 31/12/2014. Request agreed by the P- No, except if RMS AT. The substance has required nationally 20/11/1990 201309 Meda been moved to the EURD AT/H/PSUR/0038/001 201312 Work Sharing by a competent list as detailed in the