Clenbuterol Elisa Kit Instructions Product #101219 & 101216 Forensic Use Only
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Meat Biotechnology – Applications in Pork Quality Muscle Targeted Growth Promotants – Mode of Action of Beta Agonists
Meat Biotechnology – Applications in Pork Quality Muscle Targeted Growth Promotants – Mode of Action of Beta Agonists Deana Hancock, Diane Moody, Dave Anderson Elanco Animal Health Reciprocal Meat Conference, June 19, 2006 Pharmacological Activity: Beta•Adrenergic Agonist What is a beta•adrenergic agonist? A beta•adrenergic agonist (b•agonist) binds to and activates beta•adrenergic receptors (b•AR) that are found on the surface of many different types of cells in the body. G A G M L V L V G P A A A L P D G Beta Adrenergic P G L E N S S A S A T A N D P V L R A Y Receptors L L P L C K P A S P S R C P A P R C A A D S E F E S D E Y G V R E P W S S E P L T H L S R F E Q Q N F V W G F A R A P T W C R A K D A E L W Y V R G V V I W A V L (1) M (3) W H (5) I N (7) F G T T S L A L L G A S L M A F V M F V S F F A D I N L P V V P V F V L Shaded AA's are V I L C L F S V L P L W Transmembrane L G L S F V T Y W G conserved across the L I M A V L V P C Y A Domains V L A L V E I S S G A S A D A I S I C L V F T N human b1, b2 and b3 T L M A N A L W V A G M F AR's (31%) V L C V T F I N V M S C V I P I I I V I V Y G A I A L L L I A (2) F L L (4) G R (6) T Y K N D R V K C A L R F A R T T G L L C Y R R K S Q C A R R P L A F A R L E Q P D F R K A L T Q A R A E A T Q R R I K P H T L L S A R R D G A H R R T Q R L V A G L V S C Circled AA's are S K P R A L S P P Q K D conserved across F R Y I R A R G D D D D R P D V V G A D K G G N P P S the b AR of S A P G A S T 1 C A E L P humans, pigs, R L L P R P P E A F G N W R and sheep (80%) L A A A A G C G A G G A A A R P A A T D P G P P P S S S L E S K A P D D E P A S V C R F R P P G A P P P S P S P S V A Mersmann, 1998; J. -

Customs Tariff - Schedule
CUSTOMS TARIFF - SCHEDULE 99 - i Chapter 99 SPECIAL CLASSIFICATION PROVISIONS - COMMERCIAL Notes. 1. The provisions of this Chapter are not subject to the rule of specificity in General Interpretative Rule 3 (a). 2. Goods which may be classified under the provisions of Chapter 99, if also eligible for classification under the provisions of Chapter 98, shall be classified in Chapter 98. 3. Goods may be classified under a tariff item in this Chapter and be entitled to the Most-Favoured-Nation Tariff or a preferential tariff rate of customs duty under this Chapter that applies to those goods according to the tariff treatment applicable to their country of origin only after classification under a tariff item in Chapters 1 to 97 has been determined and the conditions of any Chapter 99 provision and any applicable regulations or orders in relation thereto have been met. 4. The words and expressions used in this Chapter have the same meaning as in Chapters 1 to 97. Issued January 1, 2020 99 - 1 CUSTOMS TARIFF - SCHEDULE Tariff Unit of MFN Applicable SS Description of Goods Item Meas. Tariff Preferential Tariffs 9901.00.00 Articles and materials for use in the manufacture or repair of the Free CCCT, LDCT, GPT, UST, following to be employed in commercial fishing or the commercial MT, MUST, CIAT, CT, harvesting of marine plants: CRT, IT, NT, SLT, PT, COLT, JT, PAT, HNT, Artificial bait; KRT, CEUT, UAT, CPTPT: Free Carapace measures; Cordage, fishing lines (including marlines), rope and twine, of a circumference not exceeding 38 mm; Devices for keeping nets open; Fish hooks; Fishing nets and netting; Jiggers; Line floats; Lobster traps; Lures; Marker buoys of any material excluding wood; Net floats; Scallop drag nets; Spat collectors and collector holders; Swivels. -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -
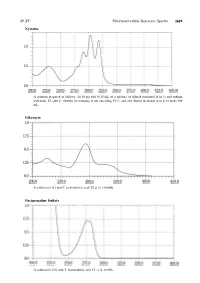
Nystatin Ofloxacin Orciprenaline Sulfate
JP XV Ultraviolet-visible Reference Spectra 1619 Nystatin A solution prepared as follows: To 10 mg add 50.25 mL of a mixture of diluted methanol (4 in 5) and sodium hydroxide TS (200:1), dissolve by warming at not exceeding 509C,andadddilutedmethanol(4in5)tomake500 mL. Ofloxacin Asolutionin0.1mol/LhydrochloricacidTS(1in150,000) Orciprenaline Sulfate A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) 1620 Ultraviolet-visible Reference Spectra JP XV Oxazolam A solution in ethanol (95) (1 in 100,000) Oxethazaine A solution in ethanol (95) (1 in 2500) Oxybuprocaine Hydrochloride An aqueous solution (1 in 100,000) JP XV Ultraviolet-visible Reference Spectra 1621 Oxycodone Hydrochloride Hydrate An aqueous solution (1 in 10,000) Oxymetholone A solution prepared as follows: To 5 mL of a solution in methanol (1 in 5000) add 5 mL of sodium hydroxide-methanol TS and methanol to make 50 mL. Oxytetracycline Hydrochloride Asolutionin0.1mol/LhydrochloricacidTS(1in50,000) 1622 Ultraviolet-visible Reference Spectra JP XV Oxytocin An aqueous solution (1 in 2000) Penbutolol Sulfate A solution in methanol (1 in 10,000) Pentazocine A solution in 0.01 mol/L hydrochloric acid TS (1 in 10,000) JP XV Ultraviolet-visible Reference Spectra 1623 Peplomycin Sulfate Asolutionpreparedasfollows:To4mgadd5mL of copper (II) sulfate TS, and dissolve in water to make 100 mL. Perphenazine 1 Asolutionin0.1mol/LhydrochloricacidTS(1in200,000) Perphenazine 2 A solution obtained by adding 10 mL of water to 10 mL of the solution for Perphenazine 1 1624 Ultraviolet-visible -
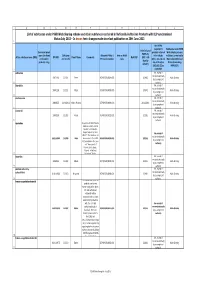
List of Substances Under PSUR WS Scheme and Other Substances
ABCDEFGHIJKL List of substances under PSUR Work Sharing scheme and other substances contained in Nationally Authorised Products with DLP synchronised Status July 2013 In brown font: changes made since last publication on 30th June 2013 1 Are PSURs required for Substances under PSUR Submission of Innovator brand products referred Work Sharing scheme PSURs by name (for fixed DLP (year Allocated PRMS / Note on PSUR to in Articles or Others (contained in Active substance name (INN) EU HBD Firm's Name Comments Next DLP (DLP + 90 combination and month) Procedure number cycle 10(1), 10a, 14 and Nationally Authorised days by products only) 16a of Directive Products including default) 2001/83/EC as MRP/DCP) 2 amended? sulbactam No, except if required nationally 19871116 201311 Pfizer AT/H/PSUR/0004/003 201402 Work Sharing by a competent 3 authority idarubicin No, except if required nationally 19891129 201311 Pfizer AT/H/PSUR/0005/003 201402 Work Sharing by a competent 4 authority dexibuprofen No, except if required nationally 20000725 31/08/2014 Gebro Pharma AT/H/PSUR/0009/003 29/11/2014 Work Sharing by a competent 5 authority nicorandil No, except if required nationally 19900209 201302 Merck AT/H/PSUR/0023/002 201305 Work Sharing by a competent 6 authority azelastine Request from MEDA Pharma GmbH & Co.KG to amend the DLP to 31/12/2014. Request agreed by the P- No, except if RMS AT. The substance has required nationally 20/11/1990 201309 Meda been moved to the EURD AT/H/PSUR/0038/001 201312 Work Sharing by a competent list as detailed in the -

Β2 Adrenergic Agonist Suppresses Eosinophil-Induced Epithelial-To- Mesenchymal Transition of Bronchial Epithelial Cells Keigo Kainuma1,2, Tetsu Kobayashi3, Corina N
Kainuma et al. Respiratory Research (2017) 18:79 DOI 10.1186/s12931-017-0563-4 RESEARCH Open Access β2 adrenergic agonist suppresses eosinophil-induced epithelial-to- mesenchymal transition of bronchial epithelial cells Keigo Kainuma1,2, Tetsu Kobayashi3, Corina N. D’Alessandro-Gabazza2, Masaaki Toda2, Taro Yasuma2, Kota Nishihama2, Hajime Fujimoto3, Yu Kuwabara1,2, Koa Hosoki1,2, Mizuho Nagao1, Takao Fujisawa1 and Esteban C. Gabazza2* Abstract Background: Epithelial-mesenchymal transition is currently recognized as an important mechanism for the increased number of myofibroblasts in cancer and fibrotic diseases. We have already reported that epithelial- mesenchymal transition is involved in airway remodeling induced by eosinophils. Procaterol is a selective and full β2 adrenergic agonist that is used as a rescue of asthmatic attack inhaler form and orally as a controller. In this study, we evaluated whether procaterol can suppress epithelial-mesenchymal transition of airway epithelial cells induced by eosinophils. Methods: Epithelial-mesenchymal transition was assessed using a co-culture system of human bronchial epithelial cells and primary human eosinophils or an eosinophilic leukemia cell line. Results: Procaterol significantly inhibited co-culture associated morphological changes of bronchial epithelial cells, decreased the expression of vimentin, and increased the expression of E-cadherin compared to control. Butoxamine, a specific β2-adrenergic antagonist, significantly blocked changes induced by procaterol. In addition, procaterol inhibited the expression of adhesion molecules induced during the interaction between eosinophils and bronchial epithelial cells, suggesting the involvement of adhesion molecules in the process of epithelial-mesenchymal transition. Forskolin, a cyclic adenosine monophosphate-promoting agent, exhibits similar inhibitory activity of procaterol. Conclusions: Overall, these observations support the beneficial effect of procaterol on airway remodeling frequently associated with chronic obstructive pulmonary diseases. -
Ep 2626065 A1
(19) TZZ Z_T (11) EP 2 626 065 A1 (12) EUROPEAN PATENT APPLICATION published in accordance with Art. 153(4) EPC (43) Date of publication: (51) Int Cl.: A61K 31/137 (2006.01) A61K 31/135 (2006.01) 14.08.2013 Bulletin 2013/33 A61K 31/4704 (2006.01) A61K 31/58 (2006.01) A61K 31/56 (2006.01) A61K 9/12 (2006.01) (2006.01) (2006.01) (21) Application number: 11827927.2 A61K 9/14 A61P 11/06 (86) International application number: Date of filing: 01.02.2011 (22) PCT/CN2011/070883 (87) International publication number: WO 2012/041031 (05.04.2012 Gazette 2012/14) (84) Designated Contracting States: (72) Inventor: WU, Wei-hsiu AL AT BE BG CH CY CZ DE DK EE ES FI FR GB Taipei GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO Taiwan (TW) PL PT RO RS SE SI SK SM TR (74) Representative: Patentanwaltskanzlei WILHELM (30) Priority: 28.09.2010 CN 201010502339 & BECK Prinzenstrasse 13 (71) Applicant: Intech Biopharm Ltd. 80639 München (DE) Taipei (TW) (54) COMPOUND COMPOSITION FOR INHALATION USED FOR TREATING ASTHMA (57) An inhaled pharmaceutical composition con- tric way as a controller. The eccentric way control therapy tains primary active ingredients of beta2- agonist and cor- could create a low blood concentration period during the ticosteroids.The pharmaceuticalcompositions disclosed day and minimize the acute tolerance phenomenon (or in the present invention are to be inhaled by a patient so called tachyphylaxis) for bronchodilator - beta2-ago- when needed as a reliever, or administrated in an eccen- nists in treating asthma or other obstructive respiratory disorders. -

That Had Torte I Una Altra Manian Literatura
THAT HAD TORTE I USUNA 20180016601A1ALTRA MANIAN LITERATURA UNA ( 19) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 / 0016601 A1 Qi et al. ( 43 ) Pub . Date: Jan . 18 , 2018 ( 54 ) METHODS FOR MODULATING GENOME C12N 15 / 10 (2006 .01 ) EDITING A6IK 38 / 46 ( 2006 .01 ) A61K 31 /365 (2006 .01 ) ( 71) Applicants: The Board of Trustees of the Leland A61K 31/ 63 ( 2006 .01 ) Stanford Junior University , Palo Alto , A61K 31 /513 ( 2006 . 01 ) CA (US ) ; The J . David Gladstone A61K 31 /505 ( 2006 .01 ) Institutes , a Testamentary Trust C12Q 1 / 68 ( 2006 .01 ) established under the Will of J . David C12N 9 / 22 ( 2006 . 01 ) Glads, San Francisco , CA ( US) ; The (52 ) U . S . CI. Regents of the University of CPC . .. - . C12N 15/ 907 ( 2013 .01 ) ; C12Q 1 /68 California , Oakland , CA (US ) ( 2013 . 01 ) ; C12Q 1 /44 ( 2013 .01 ) ; C12N 15 / 1024 (2013 .01 ) ; C12N 9 /22 ( 2013. 01 ) ; ( 72 ) Inventors : Lei S . Qi, Palo Alto , CA (US ) ; Sheng A61K 31/ 365 ( 2013 . 01) ; A61K 31 /63 Ding , Orinda , CA ( US ) ; Chen Yu , San ( 2013 .01 ) ; A61K 31/ 513 ( 2013 . 01 ) ; A61K Francisco , CA (US ) 31/ 505 ( 2013. 01 ) ; A61K 38 /465 (2013 . 01 ) (57 ) ABSTRACT ( 21 ) Appl. No. : 15 / 649, 304 Provided herein are methods and kits for modulating (22 ) Filed : Jul. 13 , 2017 genome editing of target DNA . The invention includes using small molecules that enhance or repress homology - directed Related U . S . Application Data repair (HDR ) and / or nonhomologous end joining (NHEJ ) (63 ) Continuation of application No . PCT /US2016 / repair of double - strand breaks in a target DNA sequence . -
![Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set](https://docslib.b-cdn.net/cover/8870/ehealth-dsi-ehdsi-v2-2-2-or-ehealth-dsi-master-value-set-1028870.webp)
Ehealth DSI [Ehdsi V2.2.2-OR] Ehealth DSI – Master Value Set
MTC eHealth DSI [eHDSI v2.2.2-OR] eHealth DSI – Master Value Set Catalogue Responsible : eHDSI Solution Provider PublishDate : Wed Nov 08 16:16:10 CET 2017 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 1 of 490 MTC Table of Contents epSOSActiveIngredient 4 epSOSAdministrativeGender 148 epSOSAdverseEventType 149 epSOSAllergenNoDrugs 150 epSOSBloodGroup 155 epSOSBloodPressure 156 epSOSCodeNoMedication 157 epSOSCodeProb 158 epSOSConfidentiality 159 epSOSCountry 160 epSOSDisplayLabel 167 epSOSDocumentCode 170 epSOSDoseForm 171 epSOSHealthcareProfessionalRoles 184 epSOSIllnessesandDisorders 186 epSOSLanguage 448 epSOSMedicalDevices 458 epSOSNullFavor 461 epSOSPackage 462 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 2 of 490 MTC epSOSPersonalRelationship 464 epSOSPregnancyInformation 466 epSOSProcedures 467 epSOSReactionAllergy 470 epSOSResolutionOutcome 472 epSOSRoleClass 473 epSOSRouteofAdministration 474 epSOSSections 477 epSOSSeverity 478 epSOSSocialHistory 479 epSOSStatusCode 480 epSOSSubstitutionCode 481 epSOSTelecomAddress 482 epSOSTimingEvent 483 epSOSUnits 484 epSOSUnknownInformation 487 epSOSVaccine 488 © eHealth DSI eHDSI Solution Provider v2.2.2-OR Wed Nov 08 16:16:10 CET 2017 Page 3 of 490 MTC epSOSActiveIngredient epSOSActiveIngredient Value Set ID 1.3.6.1.4.1.12559.11.10.1.3.1.42.24 TRANSLATIONS Code System ID Code System Version Concept Code Description (FSN) 2.16.840.1.113883.6.73 2017-01 A ALIMENTARY TRACT AND METABOLISM 2.16.840.1.113883.6.73 2017-01 -

Supplementary Information
1 SUPPLEMENTARY INFORMATION 2 ATIQ – further information 3 The Asthma Treatment Intrusiveness Questionnaire (ATIQ) scale was adapted from a scale originally 4 developed by Professor Horne to assess patients’ perceptions of the intrusiveness of antiretroviral 5 therapies (HAART; the HAART intrusiveness scale).1 This scale assesses convenience and the degree 6 to which the regimen is perceived by the patient to interfere with daily living, social life, etc. The 7 HAART intrusiveness scale has been applied to study differential effects of once- vs. twice-daily 8 antiretroviral regimens2 and might be usefully applied to identify patients who are most likely to 9 benefit from once-daily treatments. 10 11 References: 12 1. Newell, A., Mendes da Costa, S. & Horne, R. Assessing the psychological and therapy-related 13 barriers to optimal adherence: an observational study. Presented at the Sixth International 14 congress on Drug Therapy in HIV Infection, Glasgow, UK (2002). 15 2. Cooper, V., Horne, R., Gellaitry, G., Vrijens, B., Lange, A. C., Fisher, M. et al. The impact of once- 16 nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz- 17 based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr 53, 369–377 18 (2010). 19 1 20 Supplementary Table S1. Asthma medications, reported by participants at the time of survey Asthma medication n (%) Salbutamol 406 (40.2) Beclometasone 212 (21.0) Salmeterol plus fluticasone 209 (20.7) Salbutamol plus ipratropium 169 (16.7) Formoterol plus budesonide 166 -

GPCR/G Protein
Inhibitors, Agonists, Screening Libraries www.MedChemExpress.com GPCR/G Protein G Protein Coupled Receptors (GPCRs) perceive many extracellular signals and transduce them to heterotrimeric G proteins, which further transduce these signals intracellular to appropriate downstream effectors and thereby play an important role in various signaling pathways. G proteins are specialized proteins with the ability to bind the nucleotides guanosine triphosphate (GTP) and guanosine diphosphate (GDP). In unstimulated cells, the state of G alpha is defined by its interaction with GDP, G beta-gamma, and a GPCR. Upon receptor stimulation by a ligand, G alpha dissociates from the receptor and G beta-gamma, and GTP is exchanged for the bound GDP, which leads to G alpha activation. G alpha then goes on to activate other molecules in the cell. These effects include activating the MAPK and PI3K pathways, as well as inhibition of the Na+/H+ exchanger in the plasma membrane, and the lowering of intracellular Ca2+ levels. Most human GPCRs can be grouped into five main families named; Glutamate, Rhodopsin, Adhesion, Frizzled/Taste2, and Secretin, forming the GRAFS classification system. A series of studies showed that aberrant GPCR Signaling including those for GPCR-PCa, PSGR2, CaSR, GPR30, and GPR39 are associated with tumorigenesis or metastasis, thus interfering with these receptors and their downstream targets might provide an opportunity for the development of new strategies for cancer diagnosis, prevention and treatment. At present, modulators of GPCRs form a key area for the pharmaceutical industry, representing approximately 27% of all FDA-approved drugs. References: [1] Moreira IS. Biochim Biophys Acta. 2014 Jan;1840(1):16-33.