Supplementary Information
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers with Topical Pharmaceutical Agents
Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers With Topical Pharmaceutical Agents Author Watts, Annabelle M, Cripps, Allan W, West, Nicholas P, Cox, Amanda J Published 2019 Journal Title FRONTIERS IN PHARMACOLOGY Version Version of Record (VoR) DOI https://doi.org/10.3389/fphar.2019.00294 Copyright Statement © Frontiers in Pharmacology 2019. The attached file is reproduced here in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/386246 Griffith Research Online https://research-repository.griffith.edu.au fphar-10-00294 March 27, 2019 Time: 17:52 # 1 REVIEW published: 29 March 2019 doi: 10.3389/fphar.2019.00294 Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers With Topical Pharmaceutical Agents Annabelle M. Watts1*, Allan W. Cripps2, Nicholas P. West1 and Amanda J. Cox1 1 Menzies Health Institute Queensland, School of Medical Science, Griffith University, Southport, QLD, Australia, 2 Menzies Health Institute Queensland, School of Medicine, Griffith University, Southport, QLD, Australia Allergic rhinitis (AR) is a chronic upper respiratory disease estimated to affect between 10 and 40% of the worldwide population. The mechanisms underlying AR are highly complex and involve multiple immune cells, mediators, and cytokines. As such, the development of a single drug to treat allergic inflammation and/or symptoms is confounded by the complexity of the disease pathophysiology. Complete avoidance of allergens that trigger AR symptoms is not possible and without a cure, the available therapeutic options are typically focused on achieving symptomatic relief. -

Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis
Journal of Allergy Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis Guest Editors: Ralph Mösges, Carlos E. Baena-Cagnani, and Desiderio Passali Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis Journal of Allergy Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis Guest Editors: Ralph Mosges,¨ Carlos E. Baena-Cagnani, and Desiderio Passali Copyright © 2014 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Journal of Allergy.” All articles are open access articles distributed under the Creative Commons At- tribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board William E. Berger, USA Alan P. Knutsen, USA Fabienne Ranc, France Kurt Blaser, Switzerland Marek L. Kowalski, Poland Anuradha Ray, USA Eugene R. Bleecker, USA Ting Fan Leung, Hong Kong Harald Renz, Germany JandeMonchy,TheNetherlands Clare M Lloyd, UK Nima Rezaei, Iran Frank Hoebers, The Netherlands Redwan Moqbel, Canada Robert P. Schleimer, USA StephenT.Holgate,UK Desiderio Passali, Italy Massimo Triggiani, Italy Sebastian L. Johnston, UK Stephen P. Peters, USA Hugo Van Bever, Singapore Young J. Juhn, USA David G. Proud, Canada Garry Walsh, United Kingdom Contents Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis,RalphMosges,¨ Carlos E. Baena-Cagnani, and Desiderio Passali Volume 2014, Article ID 416236, 2 pages Clinical Efficacy of a Spray Containing Hyaluronic Acid and Dexpanthenol after Surgery in the Nasal Cavity (Septoplasty, Simple Ethmoid Sinus Surgery, and Turbinate Surgery), Ina Gouteva, Kija Shah-Hosseini, and Peter Meiser Volume 2014, Article ID 635490, 10 pages The Effectiveness of Acupuncture Compared to Loratadine in Patients Allergic to House Dust ,Mites Bettina Hauswald, Christina Dill, Jurgen¨ Boxberger, Eberhard Kuhlisch, Thomas Zahnert, and Yury M. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Doxofylline, a Novofylline Inhibits Lung Inflammation Induced By
Pulmonary Pharmacology & Therapeutics 27 (2014) 170e178 Contents lists available at ScienceDirect Pulmonary Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/ypupt Doxofylline, a novofylline inhibits lung inflammation induced by lipopolysacharide in the mouse Yanira Riffo-Vasquez*, Francis Man, Clive P. Page Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, UK article info abstract Article history: Rational: Doxofylline is a xanthine drug that has been used as a treatment for respiratory diseases for Received 20 December 2013 more than 30 years. In addition to doxofylline being a bronchodilator, some studies have indicated that Received in revised form doxofylline also has anti-inflammatory properties, although little is known about the effect of this drug 30 December 2013 on lung inflammation. Accepted 2 January 2014 Objectives: We have investigated the actions of doxofylline against the effects of Escherichia coli LPS in the lungs of BALB/c mice. Keywords: Methods: Animals have been treated with doxofylline (0.1, 0.3 and 1 mg/kg i.p.) 24, -and 1 h before, and Doxofylline m Neutrophils 6 h after intra-nasal instillation of LPS (10 g/mouse). Readouts were performed 24 h later. fi LPS Results: Doxofylline at 1 and 0.3, but not at 0.1 mg/kg, signi cantly inhibit neutrophil recruitment to the Lung lung induced by LPS (LPS: 208.4 Æ 14.5 versus doxofylline: 1 mg/kg: 106.2 Æ 4.8; 0.3 mg/kg: 4 Inflammation 105.3 Æ 10.7 Â 10 cells/ml). Doxofylline significantly inhibited IL-6 and TNF-a release into BAL fluid in Mice comparison to LPS-treated animals (LPS: 1255.6 Æ 143.9 versus doxofylline 1 mg/kg: 527.7 Æ 182.9; 0.3 mg/kg: 823.2 Æ 102.3 pg/ml). -

Impact of Doxofylline in COPD a Pairwise Meta-Analysis
King’s Research Portal DOI: 10.1016/j.pupt.2018.04.010 Document Version Publisher's PDF, also known as Version of record Link to publication record in King's Research Portal Citation for published version (APA): Cazzola, M., Calzetta, L., Rogliani, P., Page, C., & Matera, M. G. (2018). Impact of doxofylline in COPD: A pair- wise meta-analysis . PULMONARY PHARMACOLOGY AND THERAPEUTICS, 51. https://doi.org/10.1016/j.pupt.2018.04.010 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

)&F1y3x PHARMACEUTICAL APPENDIX to THE
)&f1y3X PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE )&f1y3X PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 3 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. Product CAS No. Product CAS No. ABAMECTIN 65195-55-3 ACTODIGIN 36983-69-4 ABANOQUIL 90402-40-7 ADAFENOXATE 82168-26-1 ABCIXIMAB 143653-53-6 ADAMEXINE 54785-02-3 ABECARNIL 111841-85-1 ADAPALENE 106685-40-9 ABITESARTAN 137882-98-5 ADAPROLOL 101479-70-3 ABLUKAST 96566-25-5 ADATANSERIN 127266-56-2 ABUNIDAZOLE 91017-58-2 ADEFOVIR 106941-25-7 ACADESINE 2627-69-2 ADELMIDROL 1675-66-7 ACAMPROSATE 77337-76-9 ADEMETIONINE 17176-17-9 ACAPRAZINE 55485-20-6 ADENOSINE PHOSPHATE 61-19-8 ACARBOSE 56180-94-0 ADIBENDAN 100510-33-6 ACEBROCHOL 514-50-1 ADICILLIN 525-94-0 ACEBURIC ACID 26976-72-7 ADIMOLOL 78459-19-5 ACEBUTOLOL 37517-30-9 ADINAZOLAM 37115-32-5 ACECAINIDE 32795-44-1 ADIPHENINE 64-95-9 ACECARBROMAL 77-66-7 ADIPIODONE 606-17-7 ACECLIDINE 827-61-2 ADITEREN 56066-19-4 ACECLOFENAC 89796-99-6 ADITOPRIM 56066-63-8 ACEDAPSONE 77-46-3 ADOSOPINE 88124-26-9 ACEDIASULFONE SODIUM 127-60-6 ADOZELESIN 110314-48-2 ACEDOBEN 556-08-1 ADRAFINIL 63547-13-7 ACEFLURANOL 80595-73-9 ADRENALONE -

Steroid Sparing Effects of Doxofylline
King’s Research Portal DOI: 10.1016/j.pupt.2017.10.008 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Riffo-Vasquez, Y., Venkatasamy, R., & Page, C. P. (2018). Steroid sparing effects of doxofylline. Pulmonary pharmacology & therapeutics. https://doi.org/10.1016/j.pupt.2017.10.008 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Download Product Insert (PDF)
PRODUCT INFORMATION Doxofylline Item No. 18746 CAS Registry No.: 69975-86-6 Formal Name: 7- (1, 3- dioxolan- 2- ylmethyl) - 3, 7- dihydro- O N 1, 3- dimethyl-1H- purine- 2, 6- dione N MF: C11H14N4O4 N FW: 266.3 N Purity: ≥98% O O Stability: ≥2 years at -20°C Supplied as: A crystalline solid O UV/Vis.: λmax: 273 nm Laboratory Procedures For long term storage, we suggest that doxofylline be stored as supplied at -20°C. It should be stable for at least two years. Doxofylline is supplied as a crystalline solid. A stock solution may be made by dissolving the doxofylline in the solvent of choice. Doxofylline is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of doxofylline in ethanol is approximately 0.5 mg/ml and approximately 20 mg/ml in DMSO and DMF. Doxofylline is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, doxofylline should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. Doxofylline has a solubility of approximately 0.5 mg/ml in a 1:1 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Doxofylline is a methylxanthine bronchodilator that has been examined in clinical trials involving patients with either bronchial asthma or chronic obstructive pulmonary disease.1 Its mechanism of action is related to its ability to inhibit phosphodiesterase activity and, thus, increase cAMP.2 Compared to other xanthine derivatives, which have direct arrhythmogenic effects, doxofylline demonstrates decreased affinity towards adenosine A1 and A2 receptors, does not interfere with calcium influx into cells, and does not antagonize the action of calcium-channel blockers.2,3 References 1. -

Rhinitis - Allergic (1 of 15)
Rhinitis - Allergic (1 of 15) 1 Patient presents w/ signs & symptoms of rhinitis 2 • Consider other classifi cations of rhinitis DIAGNOSIS No - Please see Rhinitis Is allergic rhinitis - Nonallergic disease confi rmed? management chart Yes 3 ASSESS DURATION & SEVERITY OF ALLERGIC RHINITIS A Non-pharmacological therapy • Allergen avoidance • Patient education VAS <5 VAS ≥5 B Pharmacological therapy B Pharmacological therapy • Antihistamines (oral/nasal), &/or • Corticosteroids (nasal), w/ or without • Corticosteroids (nasal), or • Antihistamines (nasal), or • Cromone (nasal), or • LTRA • Leukotriene receptor antagonists (LTRA)MIMS TREATMENT © See next page Specifi cally for patients w/ asthma Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B167 © MIMS Pediatrics 2020 Rhinitis - Allergic (2 of 15) Previously treated symptomatic Previously treated symptomatic patient (VAS <5) on antihistamines patient (VAS ≥5) on intensifi ed (oral/nasal) &/or corticosteroids (nasal) therapy w/ corticosteroids (nasal) w/ or without antihistamines (nasal) Intermittent Persistent symptoms, symptoms or without allergen w/ allergen exposure exposure B Pharmacological therapy B Pharmacological therapy • Step down or discontinue therapy • Continue or step up therapy Untreated REASSESS DISEASE SEVERITY VAS symptomatic patient DAILY UP TO DAY 3 (VAS <5 or ≥5) 4 CONTINUE Yes THERAPY & STEP EVALUATION VAS <5 DOWN THERAPY1 Improvement of symptoms? No VAS ≥5 B Pharmacological therapy • Step-up therapy REASSESS DISEASE SEVERITY MIMSVAS DAILY UP TO DAY 7 4 Yes EVALUATION VAS <5 Improvement of symptoms? No TREATMENT © VAS ≥5 See next page Continue therapy if symptomatic; consider step-down or discontinuation of therapy if symptoms subside Not all products are available or approved for above use in all countries. -
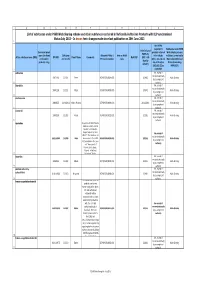
List of Substances Under PSUR WS Scheme and Other Substances
ABCDEFGHIJKL List of substances under PSUR Work Sharing scheme and other substances contained in Nationally Authorised Products with DLP synchronised Status July 2013 In brown font: changes made since last publication on 30th June 2013 1 Are PSURs required for Substances under PSUR Submission of Innovator brand products referred Work Sharing scheme PSURs by name (for fixed DLP (year Allocated PRMS / Note on PSUR to in Articles or Others (contained in Active substance name (INN) EU HBD Firm's Name Comments Next DLP (DLP + 90 combination and month) Procedure number cycle 10(1), 10a, 14 and Nationally Authorised days by products only) 16a of Directive Products including default) 2001/83/EC as MRP/DCP) 2 amended? sulbactam No, except if required nationally 19871116 201311 Pfizer AT/H/PSUR/0004/003 201402 Work Sharing by a competent 3 authority idarubicin No, except if required nationally 19891129 201311 Pfizer AT/H/PSUR/0005/003 201402 Work Sharing by a competent 4 authority dexibuprofen No, except if required nationally 20000725 31/08/2014 Gebro Pharma AT/H/PSUR/0009/003 29/11/2014 Work Sharing by a competent 5 authority nicorandil No, except if required nationally 19900209 201302 Merck AT/H/PSUR/0023/002 201305 Work Sharing by a competent 6 authority azelastine Request from MEDA Pharma GmbH & Co.KG to amend the DLP to 31/12/2014. Request agreed by the P- No, except if RMS AT. The substance has required nationally 20/11/1990 201309 Meda been moved to the EURD AT/H/PSUR/0038/001 201312 Work Sharing by a competent list as detailed in the