Impact of Doxofylline in COPD a Pairwise Meta-Analysis
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Doxofylline, a Novofylline Inhibits Lung Inflammation Induced By
Pulmonary Pharmacology & Therapeutics 27 (2014) 170e178 Contents lists available at ScienceDirect Pulmonary Pharmacology & Therapeutics journal homepage: www.elsevier.com/locate/ypupt Doxofylline, a novofylline inhibits lung inflammation induced by lipopolysacharide in the mouse Yanira Riffo-Vasquez*, Francis Man, Clive P. Page Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, UK article info abstract Article history: Rational: Doxofylline is a xanthine drug that has been used as a treatment for respiratory diseases for Received 20 December 2013 more than 30 years. In addition to doxofylline being a bronchodilator, some studies have indicated that Received in revised form doxofylline also has anti-inflammatory properties, although little is known about the effect of this drug 30 December 2013 on lung inflammation. Accepted 2 January 2014 Objectives: We have investigated the actions of doxofylline against the effects of Escherichia coli LPS in the lungs of BALB/c mice. Keywords: Methods: Animals have been treated with doxofylline (0.1, 0.3 and 1 mg/kg i.p.) 24, -and 1 h before, and Doxofylline m Neutrophils 6 h after intra-nasal instillation of LPS (10 g/mouse). Readouts were performed 24 h later. fi LPS Results: Doxofylline at 1 and 0.3, but not at 0.1 mg/kg, signi cantly inhibit neutrophil recruitment to the Lung lung induced by LPS (LPS: 208.4 Æ 14.5 versus doxofylline: 1 mg/kg: 106.2 Æ 4.8; 0.3 mg/kg: 4 Inflammation 105.3 Æ 10.7 Â 10 cells/ml). Doxofylline significantly inhibited IL-6 and TNF-a release into BAL fluid in Mice comparison to LPS-treated animals (LPS: 1255.6 Æ 143.9 versus doxofylline 1 mg/kg: 527.7 Æ 182.9; 0.3 mg/kg: 823.2 Æ 102.3 pg/ml). -

Health Reports for Mutual Recognition of Medical Prescriptions: State of Play
The information and views set out in this report are those of the author(s) and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein. Executive Agency for Health and Consumers Health Reports for Mutual Recognition of Medical Prescriptions: State of Play 24 January 2012 Final Report Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Acknowledgements Matrix Insight Ltd would like to thank everyone who has contributed to this research. We are especially grateful to the following institutions for their support throughout the study: the Pharmaceutical Group of the European Union (PGEU) including their national member associations in Denmark, France, Germany, Greece, the Netherlands, Poland and the United Kingdom; the European Medical Association (EMANET); the Observatoire Social Européen (OSE); and The Netherlands Institute for Health Service Research (NIVEL). For questions about the report, please contact Dr Gabriele Birnberg ([email protected] ). Matrix Insight | 24 January 2012 2 Health Reports for Mutual Recognition of Medical Prescriptions: State of Play Executive Summary This study has been carried out in the context of Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross- border healthcare (CBHC). The CBHC Directive stipulates that the European Commission shall adopt measures to facilitate the recognition of prescriptions issued in another Member State (Article 11). At the time of submission of this report, the European Commission was preparing an impact assessment with regards to these measures, designed to help implement Article 11. -

Steroid Sparing Effects of Doxofylline
King’s Research Portal DOI: 10.1016/j.pupt.2017.10.008 Document Version Peer reviewed version Link to publication record in King's Research Portal Citation for published version (APA): Riffo-Vasquez, Y., Venkatasamy, R., & Page, C. P. (2018). Steroid sparing effects of doxofylline. Pulmonary pharmacology & therapeutics. https://doi.org/10.1016/j.pupt.2017.10.008 Citing this paper Please note that where the full-text provided on King's Research Portal is the Author Accepted Manuscript or Post-Print version this may differ from the final Published version. If citing, it is advised that you check and use the publisher's definitive version for pagination, volume/issue, and date of publication details. And where the final published version is provided on the Research Portal, if citing you are again advised to check the publisher's website for any subsequent corrections. General rights Copyright and moral rights for the publications made accessible in the Research Portal are retained by the authors and/or other copyright owners and it is a condition of accessing publications that users recognize and abide by the legal requirements associated with these rights. •Users may download and print one copy of any publication from the Research Portal for the purpose of private study or research. •You may not further distribute the material or use it for any profit-making activity or commercial gain •You may freely distribute the URL identifying the publication in the Research Portal Take down policy If you believe that this document breaches copyright please contact [email protected] providing details, and we will remove access to the work immediately and investigate your claim. -

Download Product Insert (PDF)
PRODUCT INFORMATION Doxofylline Item No. 18746 CAS Registry No.: 69975-86-6 Formal Name: 7- (1, 3- dioxolan- 2- ylmethyl) - 3, 7- dihydro- O N 1, 3- dimethyl-1H- purine- 2, 6- dione N MF: C11H14N4O4 N FW: 266.3 N Purity: ≥98% O O Stability: ≥2 years at -20°C Supplied as: A crystalline solid O UV/Vis.: λmax: 273 nm Laboratory Procedures For long term storage, we suggest that doxofylline be stored as supplied at -20°C. It should be stable for at least two years. Doxofylline is supplied as a crystalline solid. A stock solution may be made by dissolving the doxofylline in the solvent of choice. Doxofylline is soluble in organic solvents such as ethanol, DMSO, and dimethyl formamide (DMF), which should be purged with an inert gas. The solubility of doxofylline in ethanol is approximately 0.5 mg/ml and approximately 20 mg/ml in DMSO and DMF. Doxofylline is sparingly soluble in aqueous buffers. For maximum solubility in aqueous buffers, doxofylline should first be dissolved in DMSO and then diluted with the aqueous buffer of choice. Doxofylline has a solubility of approximately 0.5 mg/ml in a 1:1 solution of DMSO:PBS (pH 7.2) using this method. We do not recommend storing the aqueous solution for more than one day. Description Doxofylline is a methylxanthine bronchodilator that has been examined in clinical trials involving patients with either bronchial asthma or chronic obstructive pulmonary disease.1 Its mechanism of action is related to its ability to inhibit phosphodiesterase activity and, thus, increase cAMP.2 Compared to other xanthine derivatives, which have direct arrhythmogenic effects, doxofylline demonstrates decreased affinity towards adenosine A1 and A2 receptors, does not interfere with calcium influx into cells, and does not antagonize the action of calcium-channel blockers.2,3 References 1. -

Theophylline Acetaldehyde As the Initial Product in Doxophylline
DMD Fast Forward. Published on February 21, 2020 as DOI: 10.1124/dmd.119.089565 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89565 Theophylline acetaldehyde as the initial product in doxophylline metabolism in human liver Xiaohua Zhao1,2, Hong Ma3,4, Qiusha Pan1,2, Haiyi Wang1,2, Xingkai Qian1,2, Peifang Song1,2, Liwei Zou1,2, Mingqing Mao5, Shuyue Xia5, Guangbo Ge1,2, Ling Yang1,2* Downloaded from 1Institute of Interdisciplinary Integrative Medicine Research, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China 2 Center for Systems Pharmacokinetics, Shanghai University of Traditional Chinese Medicine, dmd.aspetjournals.org Shanghai 201203, China 3College of Basic Medical Sciences, Dalian Medical University, Liaoning 116044, China 4Shanghai Research Institute of Acupuncture and Meridian, Shanghai 200030, China at ASPET Journals on October 2, 2021 5Respiratory Medicine Department, Central Hospital Affiliated to Shenyang Medical College, Liaoning 110034, China 1 DMD Fast Forward. Published on February 21, 2020 as DOI: 10.1124/dmd.119.089565 This article has not been copyedited and formatted. The final version may differ from this version. DMD # 89565 Running Title: Identification of the initial metabolite of doxophylline Corresponding author and contact information: Ling YANG Corresponding author at: Institute of Interdisciplinary Integrative Medicine Research, and Center for Systems Pharmacokinetics, Shanghai University of Traditional Chinese Medicine, -

Supplementary Information
1 SUPPLEMENTARY INFORMATION 2 ATIQ – further information 3 The Asthma Treatment Intrusiveness Questionnaire (ATIQ) scale was adapted from a scale originally 4 developed by Professor Horne to assess patients’ perceptions of the intrusiveness of antiretroviral 5 therapies (HAART; the HAART intrusiveness scale).1 This scale assesses convenience and the degree 6 to which the regimen is perceived by the patient to interfere with daily living, social life, etc. The 7 HAART intrusiveness scale has been applied to study differential effects of once- vs. twice-daily 8 antiretroviral regimens2 and might be usefully applied to identify patients who are most likely to 9 benefit from once-daily treatments. 10 11 References: 12 1. Newell, A., Mendes da Costa, S. & Horne, R. Assessing the psychological and therapy-related 13 barriers to optimal adherence: an observational study. Presented at the Sixth International 14 congress on Drug Therapy in HIV Infection, Glasgow, UK (2002). 15 2. Cooper, V., Horne, R., Gellaitry, G., Vrijens, B., Lange, A. C., Fisher, M. et al. The impact of once- 16 nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz- 17 based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr 53, 369–377 18 (2010). 19 1 20 Supplementary Table S1. Asthma medications, reported by participants at the time of survey Asthma medication n (%) Salbutamol 406 (40.2) Beclometasone 212 (21.0) Salmeterol plus fluticasone 209 (20.7) Salbutamol plus ipratropium 169 (16.7) Formoterol plus budesonide 166 -

Clinicaltrials.Gov Search Results 05/01/2021
ClinicalTrials.gov Search Results 09/29/2021 Title Status Study Results Conditions Interventions Locations 1 Activity, Balance and COPD (ABCOPD) Recruiting No Results Available •COPD •Other: No intervention being delivered. •National Heart and Lung Institute, Imperial College London, London, United Kingdom 2 Determination of Factors Related With Daily Living Activities in Active, not recruiting No Results Available •COPD •Bezmialem Vak#f University, #stanbul, Turkey Severe COPD 3 Impact of Specialist Led Integrated Care in COPD Active, not recruiting No Results Available •COPD •Behavioral: Intervention Arm •Dr. Sarah Pountain, Birmingham, West Midlands, United Kingdom •Behavioral: Control Arm 4 Inspiratory Muscle Training in COPD Unknown status No Results Available •COPD •Device: PrO2 •Bruce W Carter VAMC, Miami, Florida, United States •Device: Threshold Inspiratory Muscle Trainer 5 Study of Humidified Air to Improve Mucociliary Clearance Recruiting No Results Available •COPD •Device: nasal delivery of heated and •University of Pittsburgh Medical Center, Pittsburgh, (MCC) in COPD humidified air Pennsylvania, United States 6 Dose Ranging Study of RPL554 in Chronic Obstructive Completed Has Results •COPD •Drug: RPL554 suspension •Clinic for pneumonology, Pleven, Bulgaria Pulmonary Disease (COPD) Patients •Drug: Placebo •SHATPPD-Ruse EOOD, Ruse, Bulgaria •Fifth MHAT - Sofia EAD, Sofia, Bulgaria •MHAT 'Lyulin', EAD, Sofia, Bulgaria •NMTH Tsar Boris III, Sofia, Bulgaria •UMHAT 'Alexandrovska' EAD, Sofia, Bulgaria •UMHAT 'Sveta Anna' AD, Sofia, -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -
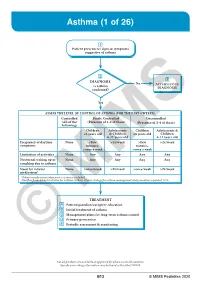
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough -

1120 Bronchodilators and Anti-Asthma Drugs
1120 Bronchodilators and Anti-asthma Drugs Profile Preparations unchanged in the urine with an elimination half-life of about 2 Cilomilast is a phosphodiesterase type-4 inhibitor that has been Proprietary Preparations (details are given in Part 3) hours. Diprophylline is distributed into breast milk. investigated in the treatment of chronic obstructive pulmonary Arg.: Bronq-C; Clembumar; Oxibron; Austria: Spiropent; Chile: Airum; disease. Uses and Administration Asmeren; Broncotosil†; Cz.: Spiropent; Ger.: Contraspasmin†; Spiropent; Diprophylline is a theophylline derivative which is used similar- Gr.: Spiropent; Hong Kong: Clenasma†; Hung.: Spiropent; Indon.: Spiropent; Ital.: Clenasma†; Monores; Prontovent†; Spiropent; Jpn: ly to theophylline (p.1146) as a bronchodilator in reversible air- Spiropent; Mex.: Novegam; Oxyflux; Spiropent; Philipp.: Spiropent; ways obstruction. Clenbuterol Hydrochloride (BANM, rINNM) ⊗ Port.: Broncoterol; Cesbron; Spain: Spiropent†; Ventolase; Venez.: Brodi- The usual oral dose of diprophylline is up to 15 mg/kg every 6 lan; Brodilin; Buclen; Clenbunal; Risopent. Clenbutérol, chlorhydrate de; Clenbuteroli hydrochloridum; Hid- hours. It has also been given intramuscularly. Diprophylline is Multi-ingredient: Arg.: Mucosolvon Compositum; Oxibron NF; Aus- rocloruro de clenbuterol; Klenbuterol hydrochlorid; Klenbuterol- also an ingredient of preparations that have been promoted for tria: Mucospas; Ger.: Spasmo-Mucosolvan; Mex.: Ambodil-C; Balsibron- coughs. hidroklorid; Klenbuterolhydroklorid; Klenbuterolihydrokloridi; -

PHARMACEUTICAL APPENDIX to the TARIFF SCHEDULE 2 Table 1
Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States (2011) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names (INN) which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service (CAS) registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Doxofylline Is Not Just Another Theophylline!
Journal name: International Journal of COPD Article Designation: Review Year: 2017 Volume: 12 International Journal of COPD Dovepress Running head verso: Matera et al Running head recto: Doxofylline tolerability profile and risk-to-benefit ratio open access to scientific and medical research DOI: http://dx.doi.org/10.2147/COPD.S150887 Open Access Full Text Article REVIEW Doxofylline is not just another theophylline! Maria Gabriella Matera1 Abstract: Doxofylline, which differs from theophylline in containing the dioxalane group at Clive Page2 position 7, has comparable efficacy to theophylline in the treatment of respiratory diseases, but Mario Cazzola3 with an improved tolerability profile and a favorable risk-to-benefit ratio. Furthermore, it does not have significant drug–drug interactions as exhibited with theophylline, which make using theo- 1Department of Experimental Medicine, Unit of Pharmacology, phylline more challenging, especially in elderly patients with co-morbidities receiving multiple University of Campania ‘Luigi classes of drug. It is now clear that doxofylline also possesses a distinct pharmacological profile 2 Vanvitelli’, Naples, Italy; Sackler from theophylline (no significant effect on any of the known phosphodiesterase isoforms, no Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, significant adenosine receptor antagonism, no direct effect on histone deacetylases, interaction King’s College London, London, with β -adrenoceptors) and therefore, should not be considered as just a modified theophylline. UK; 3Department of Experimental 2 Medicine and Surgery, Chair of Randomized clinical trials of doxofylline to investigate the use of this drug to reduce exacerba- Respiratory Medicine, University of tions and hospitalizations due to asthma or COPD as an alternative to expensive biologics, and Rome ‘Tor Vergata’, Rome, Italy certainly as an alternative to theophylline are to be encouraged.