Metered Dose Inhalers Contents
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers with Topical Pharmaceutical Agents
Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers With Topical Pharmaceutical Agents Author Watts, Annabelle M, Cripps, Allan W, West, Nicholas P, Cox, Amanda J Published 2019 Journal Title FRONTIERS IN PHARMACOLOGY Version Version of Record (VoR) DOI https://doi.org/10.3389/fphar.2019.00294 Copyright Statement © Frontiers in Pharmacology 2019. The attached file is reproduced here in accordance with the copyright policy of the publisher. Please refer to the journal's website for access to the definitive, published version. Downloaded from http://hdl.handle.net/10072/386246 Griffith Research Online https://research-repository.griffith.edu.au fphar-10-00294 March 27, 2019 Time: 17:52 # 1 REVIEW published: 29 March 2019 doi: 10.3389/fphar.2019.00294 Modulation of Allergic Inflammation in the Nasal Mucosa of Allergic Rhinitis Sufferers With Topical Pharmaceutical Agents Annabelle M. Watts1*, Allan W. Cripps2, Nicholas P. West1 and Amanda J. Cox1 1 Menzies Health Institute Queensland, School of Medical Science, Griffith University, Southport, QLD, Australia, 2 Menzies Health Institute Queensland, School of Medicine, Griffith University, Southport, QLD, Australia Allergic rhinitis (AR) is a chronic upper respiratory disease estimated to affect between 10 and 40% of the worldwide population. The mechanisms underlying AR are highly complex and involve multiple immune cells, mediators, and cytokines. As such, the development of a single drug to treat allergic inflammation and/or symptoms is confounded by the complexity of the disease pathophysiology. Complete avoidance of allergens that trigger AR symptoms is not possible and without a cure, the available therapeutic options are typically focused on achieving symptomatic relief. -

Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis
Journal of Allergy Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis Guest Editors: Ralph Mösges, Carlos E. Baena-Cagnani, and Desiderio Passali Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis Journal of Allergy Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis Guest Editors: Ralph Mosges,¨ Carlos E. Baena-Cagnani, and Desiderio Passali Copyright © 2014 Hindawi Publishing Corporation. All rights reserved. This is a special issue published in “Journal of Allergy.” All articles are open access articles distributed under the Creative Commons At- tribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. Editorial Board William E. Berger, USA Alan P. Knutsen, USA Fabienne Ranc, France Kurt Blaser, Switzerland Marek L. Kowalski, Poland Anuradha Ray, USA Eugene R. Bleecker, USA Ting Fan Leung, Hong Kong Harald Renz, Germany JandeMonchy,TheNetherlands Clare M Lloyd, UK Nima Rezaei, Iran Frank Hoebers, The Netherlands Redwan Moqbel, Canada Robert P. Schleimer, USA StephenT.Holgate,UK Desiderio Passali, Italy Massimo Triggiani, Italy Sebastian L. Johnston, UK Stephen P. Peters, USA Hugo Van Bever, Singapore Young J. Juhn, USA David G. Proud, Canada Garry Walsh, United Kingdom Contents Nonpharmacological Treatment of Rhinoconjunctivitis and Rhinosinusitis,RalphMosges,¨ Carlos E. Baena-Cagnani, and Desiderio Passali Volume 2014, Article ID 416236, 2 pages Clinical Efficacy of a Spray Containing Hyaluronic Acid and Dexpanthenol after Surgery in the Nasal Cavity (Septoplasty, Simple Ethmoid Sinus Surgery, and Turbinate Surgery), Ina Gouteva, Kija Shah-Hosseini, and Peter Meiser Volume 2014, Article ID 635490, 10 pages The Effectiveness of Acupuncture Compared to Loratadine in Patients Allergic to House Dust ,Mites Bettina Hauswald, Christina Dill, Jurgen¨ Boxberger, Eberhard Kuhlisch, Thomas Zahnert, and Yury M. -

(CD-P-PH/PHO) Report Classification/Justifica
COMMITTEE OF EXPERTS ON THE CLASSIFICATION OF MEDICINES AS REGARDS THEIR SUPPLY (CD-P-PH/PHO) Report classification/justification of medicines belonging to the ATC group R01 (Nasal preparations) Table of Contents Page INTRODUCTION 5 DISCLAIMER 7 GLOSSARY OF TERMS USED IN THIS DOCUMENT 8 ACTIVE SUBSTANCES Cyclopentamine (ATC: R01AA02) 10 Ephedrine (ATC: R01AA03) 11 Phenylephrine (ATC: R01AA04) 14 Oxymetazoline (ATC: R01AA05) 16 Tetryzoline (ATC: R01AA06) 19 Xylometazoline (ATC: R01AA07) 20 Naphazoline (ATC: R01AA08) 23 Tramazoline (ATC: R01AA09) 26 Metizoline (ATC: R01AA10) 29 Tuaminoheptane (ATC: R01AA11) 30 Fenoxazoline (ATC: R01AA12) 31 Tymazoline (ATC: R01AA13) 32 Epinephrine (ATC: R01AA14) 33 Indanazoline (ATC: R01AA15) 34 Phenylephrine (ATC: R01AB01) 35 Naphazoline (ATC: R01AB02) 37 Tetryzoline (ATC: R01AB03) 39 Ephedrine (ATC: R01AB05) 40 Xylometazoline (ATC: R01AB06) 41 Oxymetazoline (ATC: R01AB07) 45 Tuaminoheptane (ATC: R01AB08) 46 Cromoglicic Acid (ATC: R01AC01) 49 2 Levocabastine (ATC: R01AC02) 51 Azelastine (ATC: R01AC03) 53 Antazoline (ATC: R01AC04) 56 Spaglumic Acid (ATC: R01AC05) 57 Thonzylamine (ATC: R01AC06) 58 Nedocromil (ATC: R01AC07) 59 Olopatadine (ATC: R01AC08) 60 Cromoglicic Acid, Combinations (ATC: R01AC51) 61 Beclometasone (ATC: R01AD01) 62 Prednisolone (ATC: R01AD02) 66 Dexamethasone (ATC: R01AD03) 67 Flunisolide (ATC: R01AD04) 68 Budesonide (ATC: R01AD05) 69 Betamethasone (ATC: R01AD06) 72 Tixocortol (ATC: R01AD07) 73 Fluticasone (ATC: R01AD08) 74 Mometasone (ATC: R01AD09) 78 Triamcinolone (ATC: R01AD11) 82 -

Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy Using Machine Learning
Prediction of Premature Termination Codon Suppressing Compounds for Treatment of Duchenne Muscular Dystrophy using Machine Learning Kate Wang et al. Supplemental Table S1. Drugs selected by Pharmacophore-based, ML-based and DL- based search in the FDA-approved drugs database Pharmacophore WEKA TF 1-Palmitoyl-2-oleoyl-sn-glycero-3- 5-O-phosphono-alpha-D- (phospho-rac-(1-glycerol)) ribofuranosyl diphosphate Acarbose Amikacin Acetylcarnitine Acetarsol Arbutamine Acetylcholine Adenosine Aldehydo-N-Acetyl-D- Benserazide Acyclovir Glucosamine Bisoprolol Adefovir dipivoxil Alendronic acid Brivudine Alfentanil Alginic acid Cefamandole Alitretinoin alpha-Arbutin Cefdinir Azithromycin Amikacin Cefixime Balsalazide Amiloride Cefonicid Bethanechol Arbutin Ceforanide Bicalutamide Ascorbic acid calcium salt Cefotetan Calcium glubionate Auranofin Ceftibuten Cangrelor Azacitidine Ceftolozane Capecitabine Benserazide Cerivastatin Carbamoylcholine Besifloxacin Chlortetracycline Carisoprodol beta-L-fructofuranose Cilastatin Chlorobutanol Bictegravir Citicoline Cidofovir Bismuth subgallate Cladribine Clodronic acid Bleomycin Clarithromycin Colistimethate Bortezomib Clindamycin Cyclandelate Bromotheophylline Clofarabine Dexpanthenol Calcium threonate Cromoglicic acid Edoxudine Capecitabine Demeclocycline Elbasvir Capreomycin Diaminopropanol tetraacetic acid Erdosteine Carbidopa Diazolidinylurea Ethchlorvynol Carbocisteine Dibekacin Ethinamate Carboplatin Dinoprostone Famotidine Cefotetan Dipyridamole Fidaxomicin Chlormerodrin Doripenem Flavin adenine dinucleotide -

Rhinitis - Allergic (1 of 15)
Rhinitis - Allergic (1 of 15) 1 Patient presents w/ signs & symptoms of rhinitis 2 • Consider other classifi cations of rhinitis DIAGNOSIS No - Please see Rhinitis Is allergic rhinitis - Nonallergic disease confi rmed? management chart Yes 3 ASSESS DURATION & SEVERITY OF ALLERGIC RHINITIS A Non-pharmacological therapy • Allergen avoidance • Patient education VAS <5 VAS ≥5 B Pharmacological therapy B Pharmacological therapy • Antihistamines (oral/nasal), &/or • Corticosteroids (nasal), w/ or without • Corticosteroids (nasal), or • Antihistamines (nasal), or • Cromone (nasal), or • LTRA • Leukotriene receptor antagonists (LTRA)MIMS TREATMENT © See next page Specifi cally for patients w/ asthma Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B167 © MIMS Pediatrics 2020 Rhinitis - Allergic (2 of 15) Previously treated symptomatic Previously treated symptomatic patient (VAS <5) on antihistamines patient (VAS ≥5) on intensifi ed (oral/nasal) &/or corticosteroids (nasal) therapy w/ corticosteroids (nasal) w/ or without antihistamines (nasal) Intermittent Persistent symptoms, symptoms or without allergen w/ allergen exposure exposure B Pharmacological therapy B Pharmacological therapy • Step down or discontinue therapy • Continue or step up therapy Untreated REASSESS DISEASE SEVERITY VAS symptomatic patient DAILY UP TO DAY 3 (VAS <5 or ≥5) 4 CONTINUE Yes THERAPY & STEP EVALUATION VAS <5 DOWN THERAPY1 Improvement of symptoms? No VAS ≥5 B Pharmacological therapy • Step-up therapy REASSESS DISEASE SEVERITY MIMSVAS DAILY UP TO DAY 7 4 Yes EVALUATION VAS <5 Improvement of symptoms? No TREATMENT © VAS ≥5 See next page Continue therapy if symptomatic; consider step-down or discontinuation of therapy if symptoms subside Not all products are available or approved for above use in all countries. -

For Peer Review Only
BMJ Open BMJ Open: first published as 10.1136/bmjopen-2016-012177 on 30 November 2016. Downloaded from Commonly prescribed drugs associated with cognition: a cross-sectional study in UK Biobank ForJournal: peerBMJ Open review only Manuscript ID bmjopen-2016-012177 Article Type: Research Date Submitted by the Author: 06-Apr-2016 Complete List of Authors: Nevado-Holgado, Alejo; University of Oxford, Psychiatry Kim, Chi-Hun; University of Oxford, Psychiatry Winchester, Laura; University of Oxford, Psychiatry Gallacher, John; University of Oxford, Psychiatry Lovestone, Simon; University of Oxford, Psychiatry <b>Primary Subject Public health Heading</b>: Secondary Subject Heading: Mental health, Pharmacology and therapeutics, Neurology Keywords: PUBLIC HEALTH, MENTAL HEALTH, Cognition, UK biobank http://bmjopen.bmj.com/ on September 26, 2021 by guest. Protected copyright. For peer review only − http://bmjopen.bmj.com/site/about/guidelines.xhtml Page 1 of 16 BMJ Open BMJ Open: first published as 10.1136/bmjopen-2016-012177 on 30 November 2016. Downloaded from 1 2 3 Commonly prescribed drugs associate with cognition: a cross-sectional study 4 in UK Biobank 5 6 7 8 Authors 9 10 Alejo J Nevado-Holgado*, Chi-Hun Kim*, Laura Winchester, John Gallacher, Simon Lovestone 11 12 13 14 15 Address For peer review only 16 17 Department of Psychiatry, University of Oxford, Warneford Hospital, Oxford OX3 7JX, UK 18 19 20 21 22 23 Authors’ names and positions 24 25 Alejo J Nevado-Holgado*: Postdoctoral researcher 26 27 Chi-Hun Kim*: Postdoctoral researcher 28 29 Laura Winchester: Postdoctoral researcher 30 31 John Gallacher: Professor 32 33 Simon Lovestone: Professor 34 http://bmjopen.bmj.com/ 35 *These authors contributed equally to this work. -

Supplementary Information
1 SUPPLEMENTARY INFORMATION 2 ATIQ – further information 3 The Asthma Treatment Intrusiveness Questionnaire (ATIQ) scale was adapted from a scale originally 4 developed by Professor Horne to assess patients’ perceptions of the intrusiveness of antiretroviral 5 therapies (HAART; the HAART intrusiveness scale).1 This scale assesses convenience and the degree 6 to which the regimen is perceived by the patient to interfere with daily living, social life, etc. The 7 HAART intrusiveness scale has been applied to study differential effects of once- vs. twice-daily 8 antiretroviral regimens2 and might be usefully applied to identify patients who are most likely to 9 benefit from once-daily treatments. 10 11 References: 12 1. Newell, A., Mendes da Costa, S. & Horne, R. Assessing the psychological and therapy-related 13 barriers to optimal adherence: an observational study. Presented at the Sixth International 14 congress on Drug Therapy in HIV Infection, Glasgow, UK (2002). 15 2. Cooper, V., Horne, R., Gellaitry, G., Vrijens, B., Lange, A. C., Fisher, M. et al. The impact of once- 16 nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz- 17 based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr 53, 369–377 18 (2010). 19 1 20 Supplementary Table S1. Asthma medications, reported by participants at the time of survey Asthma medication n (%) Salbutamol 406 (40.2) Beclometasone 212 (21.0) Salmeterol plus fluticasone 209 (20.7) Salbutamol plus ipratropium 169 (16.7) Formoterol plus budesonide 166 -

Patent Application Publication ( 10 ) Pub . No . : US 2019 / 0192440 A1
US 20190192440A1 (19 ) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2019 /0192440 A1 LI (43 ) Pub . Date : Jun . 27 , 2019 ( 54 ) ORAL DRUG DOSAGE FORM COMPRISING Publication Classification DRUG IN THE FORM OF NANOPARTICLES (51 ) Int . CI. A61K 9 / 20 (2006 .01 ) ( 71 ) Applicant: Triastek , Inc. , Nanjing ( CN ) A61K 9 /00 ( 2006 . 01) A61K 31/ 192 ( 2006 .01 ) (72 ) Inventor : Xiaoling LI , Dublin , CA (US ) A61K 9 / 24 ( 2006 .01 ) ( 52 ) U . S . CI. ( 21 ) Appl. No. : 16 /289 ,499 CPC . .. .. A61K 9 /2031 (2013 . 01 ) ; A61K 9 /0065 ( 22 ) Filed : Feb . 28 , 2019 (2013 .01 ) ; A61K 9 / 209 ( 2013 .01 ) ; A61K 9 /2027 ( 2013 .01 ) ; A61K 31/ 192 ( 2013. 01 ) ; Related U . S . Application Data A61K 9 /2072 ( 2013 .01 ) (63 ) Continuation of application No. 16 /028 ,305 , filed on Jul. 5 , 2018 , now Pat . No . 10 , 258 ,575 , which is a (57 ) ABSTRACT continuation of application No . 15 / 173 ,596 , filed on The present disclosure provides a stable solid pharmaceuti Jun . 3 , 2016 . cal dosage form for oral administration . The dosage form (60 ) Provisional application No . 62 /313 ,092 , filed on Mar. includes a substrate that forms at least one compartment and 24 , 2016 , provisional application No . 62 / 296 , 087 , a drug content loaded into the compartment. The dosage filed on Feb . 17 , 2016 , provisional application No . form is so designed that the active pharmaceutical ingredient 62 / 170, 645 , filed on Jun . 3 , 2015 . of the drug content is released in a controlled manner. Patent Application Publication Jun . 27 , 2019 Sheet 1 of 20 US 2019 /0192440 A1 FIG . -
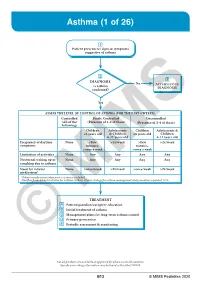
Asthma (1 of 26)
Asthma (1 of 26) 1 Patient presents w/ signs & symptoms suggestive of asthma 2 3 DIAGNOSIS No ALTERNATIVE Is asthma DIAGNOSIS confi rmed? Yes ASSESS THE LEVEL OF CONTROL OF ASTHMA FOR THE PAST 4 WEEKS Controlled Partly Controlled Uncontrolled (All of the (Presence of 1-2 of these) (Presence of 3-4 of these) following) Children Adolescents Children Adolescents & ≤5 years old & Children ≤5 years old Children 6-11 years old 6-11 years old Frequency of daytime None >Few >2x/week >Few >2x/week symptoms minutes, minutes, >once a week >once a week Limitation of activities None Any Any Any Any Nocturnal waking up or None Any Any Any Any coughing due to asthma Need for reliever None >once/week >2x/week >once/week >2x/week medication* *Reliever medications taken prior to exercise excluded. Modified from: Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention: Updated 2020. TREATMENT A Patient/guardian/caregiver education B Initial treatment of asthma C Management plans for long-term asthma control D Primary prevention E © Periodic assessmentMIMS & monitoring Not all products are available or approved for above use in all countries. Specifi c prescribing information may be found in the latest MIMS. B13 © MIMS Pediatrics 2020 Asthma (2 of 26) 1 ASTHMA • A heterogeneous disease w/ chronic infl ammatory disorder of the airways • e most common chronic disease in pediatric age groups that causes signifi cant morbidity • Characterized by history of respiratory symptoms eg wheeze, shortness of breath, chest tightness & cough -

Pharmaceutical Appendix to the Harmonized Tariff Schedule
Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE HARMONIZED TARIFF SCHEDULE Harmonized Tariff Schedule of the United States Basic Revision 3 (2021) Annotated for Statistical Reporting Purposes PHARMACEUTICAL APPENDIX TO THE TARIFF SCHEDULE 2 Table 1. This table enumerates products described by International Non-proprietary Names INN which shall be entered free of duty under general note 13 to the tariff schedule. The Chemical Abstracts Service CAS registry numbers also set forth in this table are included to assist in the identification of the products concerned. For purposes of the tariff schedule, any references to a product enumerated in this table includes such product by whatever name known. -

Vr Meds Ex01 3B 0825S Coding Manual Supplement Page 1
vr_meds_ex01_3b_0825s Coding Manual Supplement MEDNAME OTHER_CODE ATC_CODE SYSTEM THER_GP PHRM_GP CHEM_GP SODIUM FLUORIDE A12CD01 A01AA01 A A01 A01A A01AA SODIUM MONOFLUOROPHOSPHATE A12CD02 A01AA02 A A01 A01A A01AA HYDROGEN PEROXIDE D08AX01 A01AB02 A A01 A01A A01AB HYDROGEN PEROXIDE S02AA06 A01AB02 A A01 A01A A01AB CHLORHEXIDINE B05CA02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D08AC02 A01AB03 A A01 A01A A01AB CHLORHEXIDINE D09AA12 A01AB03 A A01 A01A A01AB CHLORHEXIDINE R02AA05 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S01AX09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S02AA09 A01AB03 A A01 A01A A01AB CHLORHEXIDINE S03AA04 A01AB03 A A01 A01A A01AB AMPHOTERICIN B A07AA07 A01AB04 A A01 A01A A01AB AMPHOTERICIN B G01AA03 A01AB04 A A01 A01A A01AB AMPHOTERICIN B J02AA01 A01AB04 A A01 A01A A01AB POLYNOXYLIN D01AE05 A01AB05 A A01 A01A A01AB OXYQUINOLINE D08AH03 A01AB07 A A01 A01A A01AB OXYQUINOLINE G01AC30 A01AB07 A A01 A01A A01AB OXYQUINOLINE R02AA14 A01AB07 A A01 A01A A01AB NEOMYCIN A07AA01 A01AB08 A A01 A01A A01AB NEOMYCIN B05CA09 A01AB08 A A01 A01A A01AB NEOMYCIN D06AX04 A01AB08 A A01 A01A A01AB NEOMYCIN J01GB05 A01AB08 A A01 A01A A01AB NEOMYCIN R02AB01 A01AB08 A A01 A01A A01AB NEOMYCIN S01AA03 A01AB08 A A01 A01A A01AB NEOMYCIN S02AA07 A01AB08 A A01 A01A A01AB NEOMYCIN S03AA01 A01AB08 A A01 A01A A01AB MICONAZOLE A07AC01 A01AB09 A A01 A01A A01AB MICONAZOLE D01AC02 A01AB09 A A01 A01A A01AB MICONAZOLE G01AF04 A01AB09 A A01 A01A A01AB MICONAZOLE J02AB01 A01AB09 A A01 A01A A01AB MICONAZOLE S02AA13 A01AB09 A A01 A01A A01AB NATAMYCIN A07AA03 A01AB10 A A01 -

Alphabetical Listing of ATC Drugs & Codes
Alphabetical Listing of ATC drugs & codes. Introduction This file is an alphabetical listing of ATC codes as supplied to us in November 1999. It is supplied free as a service to those who care about good medicine use by mSupply support. To get an overview of the ATC system, use the “ATC categories.pdf” document also alvailable from www.msupply.org.nz Thanks to the WHO collaborating centre for Drug Statistics & Methodology, Norway, for supplying the raw data. I have intentionally supplied these files as PDFs so that they are not quite so easily manipulated and redistributed. I am told there is no copyright on the files, but it still seems polite to ask before using other people’s work, so please contact <[email protected]> for permission before asking us for text files. mSupply support also distributes mSupply software for inventory control, which has an inbuilt system for reporting on medicine usage using the ATC system You can download a full working version from www.msupply.org.nz Craig Drown, mSupply Support <[email protected]> April 2000 A (2-benzhydryloxyethyl)diethyl-methylammonium iodide A03AB16 0.3 g O 2-(4-chlorphenoxy)-ethanol D01AE06 4-dimethylaminophenol V03AB27 Abciximab B01AC13 25 mg P Absorbable gelatin sponge B02BC01 Acadesine C01EB13 Acamprosate V03AA03 2 g O Acarbose A10BF01 0.3 g O Acebutolol C07AB04 0.4 g O,P Acebutolol and thiazides C07BB04 Aceclidine S01EB08 Aceclidine, combinations S01EB58 Aceclofenac M01AB16 0.2 g O Acefylline piperazine R03DA09 Acemetacin M01AB11 Acenocoumarol B01AA07 5 mg O Acepromazine N05AA04