COVID-19 Evidence Bulletin 8
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Diagnosis and Management of Asthma in Older Adults Sanjay Haresh Chotirmall, MD, Michael Watts, MD, Peter Branagan, MD, Ciaran F
PROGRESS IN GERIATRICS Diagnosis and Management of Asthma in Older Adults Sanjay Haresh Chotirmall, MD, Michael Watts, MD, Peter Branagan, MD, Ciaran F. Donegan, MD, Allan Moore, MD, and Noel Gerard McElvaney, MD Despite comprehensive guidelines established by the Euro- from 6.5% to 17.0%.5 Death rates associated with asthma pean Global Initiative for Asthma and the U.S. National depend on patient age; in a group of patients aged 55 to 59, Asthma Education and Prevention Program on the diagno- the death rate was 2.8 per 100,000 people, whereas in sis and management of asthma, its mortality in older adults people aged 60 to 64, it was 4.2 per 100, 000.6 Diagnostic continues to rise. Diagnostic and therapeutic problems and therapeutic problems contribute to many patients being contribute to older patients being inadequately treated. The inadequately treated. Despite its importance in older pa- diagnosis of asthma rests on the history and characteristic tients, asthma is particularly difficult to diagnose in this age pulmonary function testing (PFT) with the demonstration group. Symptoms typical of asthma such as intermittent of reversible airway obstruction, but there are unique prob- wheezing, breathlessness, and cough can also indicate other lems in performing this test in older patients and in its in- respiratory problems in older patients, particularly chronic terpretation. This review aims to address the difficulties in obstructive pulmonary disease (COPD). Similarly, other performing and interpreting PFT in older patients because symptoms of asthma such as chest pain or tightness may of the effects of age-related changes in lung function on be due to nonpulmonary disease such as ischemic heart respiratory physiology. -

Emea/666243/2009
European Medicines Agency London, 29 October 2009 EMEA/666243/2009 ISSUE NUMBER: 0910 MONTHLY REPORT PHARMACOVIGILANCE WORKING PARTY (PHVWP) OCTOBER 2009 PLENARY MEETING The CHMP Pharmacovigilance Working Party (PhVWP) held its October 2009 plenary meeting on 19-21 October 2009. PhVWP DISCUSSIONS ON SAFETY CONCERNS Below is a summary of the discussions regarding non-centrally authorised medicinal products in accordance with the PhVWP publication policy (see under http://www.emea.europa.eu/htms/human/phv/reports.htm). Positions agreed by the PhVWP for non- centrally authorised products are recommendations to Member States. For safety updates concerning centrally authorised products and products subject to ongoing CHMP procedures, readers are referred to the CHMP Monthly Report (see under http://www.emea.europa.eu/pressoffice/presshome.htm). The PhVWP provides advice on these products to the Committee of Medicinal Products for Human Use (CHMP) upon its request. Antipsychotics - risk of venous thromboembolism (VTE) Identify risk factors for VTE for preventive action before and during treatment with antipsychotics The PhVWP completed their review on the risk of VTE of antipsychotics1. The review was triggered by and based on data from the UK spontaneous adverse drug reactions reporting system and the published literature. The PhVWP carefully considered the data, including the limitations of both information sources, such as the lack of randomised controlled trial data, the heterogeneity of published studies and the potential confounding factors such as sedation and weight gain, commonly present in antipsychotic users. The PhVWP concluded that an association between VTE and antipsychotics cannot be excluded. Distinguishing different risk levels between the various active substances was not possible. -

Lamas for COPD Systematic Review
Comparative safety and effectiveness of inhaled long -acting agents (corticosteroids, beta agonists, anticholinergics) for chronic obstructive pulmonary disease Comprehensive Research Plan: Systematic Review Unit April 4th, 2014 Andrea C. Tricco, PhD1 and Sharon E. Straus, MD, MSc1,2 30 Bond Street, Toronto ON, M5B 1W8 www.odprn.ca [email protected] Background Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation in the lungs [1]. COPD is commonly assessed by clinical examination and spirometry. Important indicators considered in the diagnosis of COPD include age over 40 years and any of the following: 1) progressive and persistent dyspnea that worsens with exercise, 2) chronic cough, 3) chronic sputum production, 4) history of exposure to smoke from tobacco or cooking, occupational dusts and chemicals, and 5) family history of COPD [1]. COPD causes significant burden of illness, reduced quality of life, and premature death [2]. Symptoms include chronic cough, sputum production, and dyspnea [3]. The global prevalence of COPD has been estimated at 7.6% using data from a systematic review including 28 countries [4]. However, this is likely a conservative estimate, due to under-reporting and under-diagnosis. The prevalence and burden of COPD is rising due the greater proportion of elderly people in the population [1]. It is estimated that COPD will be the third-leading cause of death by 2020 [5]. The treatment of COPD usually involves reducing exposure (e.g., smoking cessation, occupation modifications), increasing exercise, and implementing appropriate pharmacologic therapy [1]. The most common drug classes are beta2-agonists, anticholinergics, and methylxanthines. Inhaled corticosteroids (ICS) and systemic corticosteroids are often useful for acute exacerbations. -
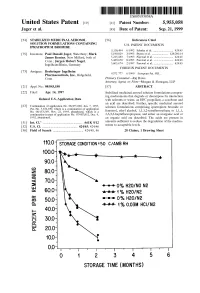
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int. -

Pharmacology and Therapeutics of Bronchodilators
1521-0081/12/6403-450–504$25.00 PHARMACOLOGICAL REVIEWS Vol. 64, No. 3 Copyright © 2012 by The American Society for Pharmacology and Experimental Therapeutics 4580/3762238 Pharmacol Rev 64:450–504, 2012 ASSOCIATE EDITOR: DAVID R. SIBLEY Pharmacology and Therapeutics of Bronchodilators Mario Cazzola, Clive P. Page, Luigino Calzetta, and M. Gabriella Matera Department of Internal Medicine, Unit of Respiratory Clinical Pharmacology, University of Rome ‘Tor Vergata,’ Rome, Italy (M.C., L.C.); Department of Pulmonary Rehabilitation, San Raffaele Pisana Hospital, Istituto di Ricovero e Cura a Carattere Scientifico, Rome, Italy (M.C., L.C.); Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, London, UK (C.P.P., L.C.); and Department of Experimental Medicine, Unit of Pharmacology, Second University of Naples, Naples, Italy (M.G.M.) Abstract............................................................................... 451 I. Introduction: the physiological rationale for using bronchodilators .......................... 452 II. -Adrenergic receptor agonists .......................................................... 455 A. A history of the development of -adrenergic receptor agonists: from nonselective  Downloaded from adrenergic receptor agonists to 2-adrenergic receptor-selective drugs.................... 455  B. Short-acting 2-adrenergic receptor agonists........................................... 457 1. Albuterol........................................................................ 457 -

Bnf Chapter 3: Respiratory
BNF CHAPTER 3: RESPIRATORY Information resources: ● If an inhaler device is changed then patients must be assessed to ensure inhaler technique is adequate Respiratory Futures Consider issuing a Steroid warning card to patients prescribed high dose inhaled steroids Asthma guidelines NICE guideline [NG80] Asthma: diagnosis, monitoring and chronic asthma management NICE guidance TA 138 Asthma - inhaled corticosteroids for the treatment of chronic asthma in adults and children aged 12 years and over. https://www.sign.ac.uk/assets/sign153.pdf SIGN 153: British guideline on the management of asthma Global Initiative for Asthma 2018. GINA Report, Global Strategy for Asthma Management and Prevention. West Cheshire local guidelines COPD guidelines: NICE [NG115] COPD in over 16s diagnosis and management. NICE guideline Dec 18 [NG115] GOLD : Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease 2018 Report West Cheshire local guidelines Abbreviations used: MDI: metered dose inhaler DPI: dry powder inhaler SABA: short acting beta agonist LABA: long acting beta agonist SAMA: short acting muscarinic antagonist LAMA: long acting muscarinic antagonist ICS: inhaled corticosteroid LTRA: leukotriene receptor antagonist PDE4: phosphodiesterase-4 enzyme inhibitor Joint Formulary – Respiratory Approved by Area Prescribing Committee: n/a Review by: July 2021 3.1 BRONCHODILATORS 3.1.1.1 SELECTIVE BETA2-AGONISTS Short acting Beta2 Agonist (SABA) Salbutamol 100 micrograms/metered inhalation MDI 100/200 -

Dry Powder Inhalers Comprising a Carrier Other Than Lactose
(19) TZZ Z T (11) EP 2 682 097 A2 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 08.01.2014 Bulletin 2014/02 A61K 9/00 (2006.01) A61K 9/14 (2006.01) A61K 31/40 (2006.01) A61K 31/439 (2006.01) (2006.01) (21) Application number: 13175077.0 A61K 31/4704 (22) Date of filing: 04.07.2013 (84) Designated Contracting States: • Türkyilmaz, Ali AL AT BE BG CH CY CZ DE DK EE ES FI FR GB 34460 Istanbul (TR) GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • Mutlu, Onur PL PT RO RS SE SI SK SM TR 34460 Istanbul (TR) Designated Extension States: BA ME (74) Representative: Sevinç, Erkan Istanbul Patent & Trademark (30) Priority: 05.07.2012 TR 201207842 Consultancy Ltd. Plaza 33, Buyukdere Cad. No: 33/16 (71) Applicant: Arven Ilac Sanayi Ve Ticaret A.S. Sisli Istanbul 34460 (TR) 34381 Istanbul (TR) (72) Inventors: • Cifter, Ümit 34460 Istanbul (TR) (54) Dry Powder Inhalers Comprising A Carrier Other Than Lactose (57) This invention relates to novel pharmaceutical other obstructive airways diseases. In addition, the compositions for inhalation comprising separately, se- present invention relates to novel pharmaceutical com- quentially or together, drugs having amine in the form of position for inhalation based on combinations of long act- a dry powder in admixture with a pharmaceutically ac- ing muscarinic antagonists, long acting beta agonists, ceptable carrier, other than lactose, and its use in the short acting beta-2 agonists, corticosteroids or a combi- treatment of respiratory condition selected from asthma nation of two or more of them. -

Management of Asthma in Athletes Michael G
Journal of Athletic Training 2005;40(3):224±245 q by the National Athletic Trainers' Association, Inc www.journalofathletictraining.org National Athletic Trainers' Association Position Statement: Management of Asthma in Athletes Michael G. Miller*; John M. Weiler²; Robert Baker³; James Collins§; Gilbert D'Alonzo\ *Western Michigan University, Kalamazoo, MI; ²University of Iowa and CompleWare, Iowa City, IA; ³Michigan State University Kalamazoo Center for Medical Studies, Kalamazoo, MI; §San Diego Chargers, San Diego, CA; \Temple University School of Medicine, Philadelphia, PA Michael G. Miller, EdD, ATC, CSCS; John M. Weiler, MD; Robert Baker, MD, PhD, ATC; James Collins, ATC; and Gilbert D'Alonzo, DO, contributed to conception and design; acquisition and analysis and interpretation of the data; and drafting, critical revision, and ®nal approval of the article. Address correspondence to National Athletic Trainers' Association, Communications Department, 2952 Stemmons Freeway, Dallas, TX 75247. Objective: To present guidelines for the recognition, prophy- in supervising therapies to prevent and control asthma symp- laxis, and management of asthma that lead to improvement in toms. It is also important for the athletic trainer to recognize the quality of care certi®ed athletic trainers and other heath care when asthma is not the underlying cause for respiratory dif®- providers can offer to athletes with asthma, especially exercise- culties, so that the athlete can be evaluated and treated prop- induced asthma. erly. Background: Many athletes have dif®culty breathing during Recommendations: The recommendations contained in this or after athletic events and practices. Although a wide variety position statement describe a structured approach for the di- of conditions can predispose an athlete to breathing dif®culties, agnosis and management of asthma in an exercising popula- tion. -

(12) Patent Application Publication (10) Pub. No.: US 2014/0302147 A1 HARTMAN Et Al
US 20140302147A1 (19) United States (12) Patent Application Publication (10) Pub. No.: US 2014/0302147 A1 HARTMAN et al. (43) Pub. Date: Oct. 9, 2014 (54) RESPRABLEAGGLOMERATES OF POROUS Publication Classification CARRIER PARTICLES AND MICRONIZED DRUG (51) Int. Cl. A619/16 (2006.01) (71) Applicants: Michael HARTMAN, Millbrae, CA A619/00 (2006.01) (US); Thomas ETARARA, A613 L/40 (2006.01) Burlingame, CA (US); Patrick TEUNG, A613 L/4704 (2006.01) Sunnyvale, CA (US); Jeffry G WEERS, A613 L/58 (2006.01) Belmont, CA (US) (52) U.S. Cl. CPC ........... A61 K9/1617 (2013.01); A61 K3I/4704 (72) Inventors: Michael HARTMAN, Millbrae, CA (2013.01); A61 K3I/58 (2013.01); A61K 31/40 (US); Thomas ETARARA, (2013.01); A61 K9/1682 (2013.01); A61 K Burlingame, CA (US); Patrick TEUNG, 9/0075 (2013.01) Sunnyvale, CA (US); Jeffry G WEERS, USPC ............................ 424/489: 514/312: 514/171 Belmont, CA (US) (57) ABSTRACT Embodiments of the present invention provide a dry powder Assignee: composition comprising porous carrier particles associated (73) NOVARTIS AG, Basel (CH) with one, two, three or more micronized drugs or APIs wherein an ordered mixture between the micronized drug or (21) Appl. No.: 14/202,262 drugs and the carrier particle results, such that the micronized drug or drugs adhere strongly to the carrier particles forming a stable ordered mixture of respirable agglomerates. Embodi (22) Filed: Mar 10, 2014 ments of the present invention further comprise a spray-dry ing process to create the respirable agglomerates. Embodi ments of the present invention further relate to the use of the Related U.S. -

Supplementary Material A: RECORD Statement
BMJ Publishing Group Limited (BMJ) disclaims all liability and responsibility arising from any reliance Supplemental material placed on this supplemental material which has been supplied by the author(s) BMJ Open Supplementary Material A: RECORD Statement The RECORD statement – checklist of items, extended from the STROBE statement, that should be reported in observational studies using routinely collected health data. Item STROBE items Location in RECORD items Location in manuscript where No. manuscript where items are reported items are reported Title and abstract 1 (a) Indicate the Page 1 in title and RECORD 1.1: The type of data 1.1 Page 1 in the abstract. study’s design with abstract used should be specified in the a commonly used title or abstract. When possible, term in the title or the name of the databases used the abstract (b) should be included. Provide in the 1.2 Page 1 in the abstract. abstract an RECORD 1.2: If applicable, informative and the geographic region and balanced summary timeframe within which the of what was done study took place should be and what was found reported in the title or abstract. 1.3 Page 1 – data were linked in a system wide dataset. RECORD 1.3: If linkage between databases was conducted for the study, this should be clearly stated in the title or abstract. Introduction Background 2 Explain the Page 3 under rationale scientific Introduction background and rationale for the investigation being reported Objectives 3 State specific Page 3 at the bottom objectives, including of the page. any prespecified hypotheses Methods Study Design 4 Present key elements Page 4 under 2.1 Data, of study design early application and in the paper setting. -

Stembook 2018.Pdf
The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 WHO/EMP/RHT/TSN/2018.1 © World Health Organization 2018 Some rights reserved. This work is available under the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 IGO licence (CC BY-NC-SA 3.0 IGO; https://creativecommons.org/licenses/by-nc-sa/3.0/igo). Under the terms of this licence, you may copy, redistribute and adapt the work for non-commercial purposes, provided the work is appropriately cited, as indicated below. In any use of this work, there should be no suggestion that WHO endorses any specific organization, products or services. The use of the WHO logo is not permitted. If you adapt the work, then you must license your work under the same or equivalent Creative Commons licence. If you create a translation of this work, you should add the following disclaimer along with the suggested citation: “This translation was not created by the World Health Organization (WHO). WHO is not responsible for the content or accuracy of this translation. The original English edition shall be the binding and authentic edition”. Any mediation relating to disputes arising under the licence shall be conducted in accordance with the mediation rules of the World Intellectual Property Organization. Suggested citation. The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances. Geneva: World Health Organization; 2018 (WHO/EMP/RHT/TSN/2018.1). Licence: CC BY-NC-SA 3.0 IGO. Cataloguing-in-Publication (CIP) data.