Comparison Between a New Once-Daily, Bronchodilating Drug, Bambuterol, and Terbutaline Sustained-Release, Twice Daily
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

Ep 2560611 B1
(19) TZZ Z___T (11) EP 2 560 611 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: A61K 9/00 (2006.01) 03.01.2018 Bulletin 2018/01 (86) International application number: (21) Application number: 11719211.2 PCT/EP2011/056227 (22) Date of filing: 19.04.2011 (87) International publication number: WO 2011/131663 (27.10.2011 Gazette 2011/43) (54) "PROCESS FOR PROVIDING PARTICLES WITH REDUCED ELECTROSTATIC CHARGES" VERFAHREN ZUR BEREITSTELLUNG VON PARTIKELN MIT REDUZIERTEN ELEKTROSTATISCHEN LADUNGEN PROCÉDÉ DE PRÉPARATION DE PARTICULES AYANT DES CHARGES ÉLECTROSTATIQUES RÉDUITES (84) Designated Contracting States: • GUCHARDI ET AL: "Influence of fine lactose and AL AT BE BG CH CY CZ DE DK EE ES FI FR GB magnesium stearate on low dose dry powder GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO inhaler formulations", INTERNATIONAL PL PT RO RS SE SI SK SM TR JOURNAL OF PHARMACEUTICS, ELSEVIER BV, NL LNKD- DOI:10.1016/J.IJPHARM.2007.06.041, (30) Priority: 21.04.2010 EP 10160565 vol. 348, no. 1-2, 19 December 2007 (2007-12-19), pages 10-17, XP022393884, ISSN: 0378-5173 (43) Date of publication of application: • ELAJNAF A ET AL: "Electrostatic 27.02.2013 Bulletin 2013/09 characterisation of inhaled powders: Effect of contact surface and relative humidity", (73) Proprietor: Chiesi Farmaceutici S.p.A. EUROPEAN JOURNAL OF PHARMACEUTICAL 43100 Parma (IT) SCIENCES, ELSEVIER, AMSTERDAM, NL LNKD- DOI:10.1016/J.EJPS.2006.07.006, vol. 29, no. 5, 1 (72) Inventors: December 2006 (2006-12-01), pages 375-384, • COCCONI, Daniela XP025137181, ISSN: 0928-0987 [retrieved on I-43100 Parma (IT) 2006-12-01] • MUSA, Rossella • CHAN ET AL: "Dry powder aerosol drug I-43100 Parma (IT) delivery-Opportunities for colloid and surface scientists", COLLOIDS AND SURFACES. -

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

COVID-19 Evidence Bulletin 8
COVID-19 Evidence Bulletin 8 Public Health England PHE International Epidemiology Daily Evidence Digest – 22nd April 2020 – 21st April 2020 – 20th April 2020 NICE COVID-19 rapid guideline: acute myocardial injury [NG171] Published 23rd April The purpose of this guideline is to help healthcare professionals who are not cardiology specialists identify and treat acute myocardial injury and its cardiac complications in adults with known or suspected COVID-19 but without known pre-existing cardiovascular disease. COVID-19 rapid guideline: gastrointestinal and liver conditions treated with drugs affecting the immune response [NG172] Published 23rd April The purpose of this guideline is to maximise the safety of children and adults who have gastrointestinal or liver conditions treated with drugs affecting the immune response during the COVID 19 pandemic. It also aims to protect staff from infection and enable services to make the best use of NHS resources. COVID-19 rapid guideline: managing symptoms (including at the end of life) in the community Published 3rd April, Last updated 22nd April NHS England Specialty Guides: Clinical guide for acute kidney injury in hospitalised patients with COVID-19 outside the intensive care unit during the coronavirus pandemic (22nd April - updated) Management of palliative care in hospital during the coronavirus pandemic (22nd April – updated) Department of Health and Social Care Medicines that cannot be parallel exported from the UK (22nd April) 33 medicines have been added to the parallel export list and the -

Diagnosis and Management of Asthma in Older Adults Sanjay Haresh Chotirmall, MD, Michael Watts, MD, Peter Branagan, MD, Ciaran F
PROGRESS IN GERIATRICS Diagnosis and Management of Asthma in Older Adults Sanjay Haresh Chotirmall, MD, Michael Watts, MD, Peter Branagan, MD, Ciaran F. Donegan, MD, Allan Moore, MD, and Noel Gerard McElvaney, MD Despite comprehensive guidelines established by the Euro- from 6.5% to 17.0%.5 Death rates associated with asthma pean Global Initiative for Asthma and the U.S. National depend on patient age; in a group of patients aged 55 to 59, Asthma Education and Prevention Program on the diagno- the death rate was 2.8 per 100,000 people, whereas in sis and management of asthma, its mortality in older adults people aged 60 to 64, it was 4.2 per 100, 000.6 Diagnostic continues to rise. Diagnostic and therapeutic problems and therapeutic problems contribute to many patients being contribute to older patients being inadequately treated. The inadequately treated. Despite its importance in older pa- diagnosis of asthma rests on the history and characteristic tients, asthma is particularly difficult to diagnose in this age pulmonary function testing (PFT) with the demonstration group. Symptoms typical of asthma such as intermittent of reversible airway obstruction, but there are unique prob- wheezing, breathlessness, and cough can also indicate other lems in performing this test in older patients and in its in- respiratory problems in older patients, particularly chronic terpretation. This review aims to address the difficulties in obstructive pulmonary disease (COPD). Similarly, other performing and interpreting PFT in older patients because symptoms of asthma such as chest pain or tightness may of the effects of age-related changes in lung function on be due to nonpulmonary disease such as ischemic heart respiratory physiology. -

That Had Torte I Una Altra Manian Literatura
THAT HAD TORTE I USUNA 20180016601A1ALTRA MANIAN LITERATURA UNA ( 19) United States (12 ) Patent Application Publication ( 10) Pub . No. : US 2018 / 0016601 A1 Qi et al. ( 43 ) Pub . Date: Jan . 18 , 2018 ( 54 ) METHODS FOR MODULATING GENOME C12N 15 / 10 (2006 .01 ) EDITING A6IK 38 / 46 ( 2006 .01 ) A61K 31 /365 (2006 .01 ) ( 71) Applicants: The Board of Trustees of the Leland A61K 31/ 63 ( 2006 .01 ) Stanford Junior University , Palo Alto , A61K 31 /513 ( 2006 . 01 ) CA (US ) ; The J . David Gladstone A61K 31 /505 ( 2006 .01 ) Institutes , a Testamentary Trust C12Q 1 / 68 ( 2006 .01 ) established under the Will of J . David C12N 9 / 22 ( 2006 . 01 ) Glads, San Francisco , CA ( US) ; The (52 ) U . S . CI. Regents of the University of CPC . .. - . C12N 15/ 907 ( 2013 .01 ) ; C12Q 1 /68 California , Oakland , CA (US ) ( 2013 . 01 ) ; C12Q 1 /44 ( 2013 .01 ) ; C12N 15 / 1024 (2013 .01 ) ; C12N 9 /22 ( 2013. 01 ) ; ( 72 ) Inventors : Lei S . Qi, Palo Alto , CA (US ) ; Sheng A61K 31/ 365 ( 2013 . 01) ; A61K 31 /63 Ding , Orinda , CA ( US ) ; Chen Yu , San ( 2013 .01 ) ; A61K 31/ 513 ( 2013 . 01 ) ; A61K Francisco , CA (US ) 31/ 505 ( 2013. 01 ) ; A61K 38 /465 (2013 . 01 ) (57 ) ABSTRACT ( 21 ) Appl. No. : 15 / 649, 304 Provided herein are methods and kits for modulating (22 ) Filed : Jul. 13 , 2017 genome editing of target DNA . The invention includes using small molecules that enhance or repress homology - directed Related U . S . Application Data repair (HDR ) and / or nonhomologous end joining (NHEJ ) (63 ) Continuation of application No . PCT /US2016 / repair of double - strand breaks in a target DNA sequence . -

The Use of Stems in the Selection of International Nonproprietary Names (INN) for Pharmaceutical Substances
WHO/PSM/QSM/2006.3 The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances 2006 Programme on International Nonproprietary Names (INN) Quality Assurance and Safety: Medicines Medicines Policy and Standards The use of stems in the selection of International Nonproprietary Names (INN) for pharmaceutical substances FORMER DOCUMENT NUMBER: WHO/PHARM S/NOM 15 © World Health Organization 2006 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. -

Emea/666243/2009
European Medicines Agency London, 29 October 2009 EMEA/666243/2009 ISSUE NUMBER: 0910 MONTHLY REPORT PHARMACOVIGILANCE WORKING PARTY (PHVWP) OCTOBER 2009 PLENARY MEETING The CHMP Pharmacovigilance Working Party (PhVWP) held its October 2009 plenary meeting on 19-21 October 2009. PhVWP DISCUSSIONS ON SAFETY CONCERNS Below is a summary of the discussions regarding non-centrally authorised medicinal products in accordance with the PhVWP publication policy (see under http://www.emea.europa.eu/htms/human/phv/reports.htm). Positions agreed by the PhVWP for non- centrally authorised products are recommendations to Member States. For safety updates concerning centrally authorised products and products subject to ongoing CHMP procedures, readers are referred to the CHMP Monthly Report (see under http://www.emea.europa.eu/pressoffice/presshome.htm). The PhVWP provides advice on these products to the Committee of Medicinal Products for Human Use (CHMP) upon its request. Antipsychotics - risk of venous thromboembolism (VTE) Identify risk factors for VTE for preventive action before and during treatment with antipsychotics The PhVWP completed their review on the risk of VTE of antipsychotics1. The review was triggered by and based on data from the UK spontaneous adverse drug reactions reporting system and the published literature. The PhVWP carefully considered the data, including the limitations of both information sources, such as the lack of randomised controlled trial data, the heterogeneity of published studies and the potential confounding factors such as sedation and weight gain, commonly present in antipsychotic users. The PhVWP concluded that an association between VTE and antipsychotics cannot be excluded. Distinguishing different risk levels between the various active substances was not possible. -

Lamas for COPD Systematic Review
Comparative safety and effectiveness of inhaled long -acting agents (corticosteroids, beta agonists, anticholinergics) for chronic obstructive pulmonary disease Comprehensive Research Plan: Systematic Review Unit April 4th, 2014 Andrea C. Tricco, PhD1 and Sharon E. Straus, MD, MSc1,2 30 Bond Street, Toronto ON, M5B 1W8 www.odprn.ca [email protected] Background Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation in the lungs [1]. COPD is commonly assessed by clinical examination and spirometry. Important indicators considered in the diagnosis of COPD include age over 40 years and any of the following: 1) progressive and persistent dyspnea that worsens with exercise, 2) chronic cough, 3) chronic sputum production, 4) history of exposure to smoke from tobacco or cooking, occupational dusts and chemicals, and 5) family history of COPD [1]. COPD causes significant burden of illness, reduced quality of life, and premature death [2]. Symptoms include chronic cough, sputum production, and dyspnea [3]. The global prevalence of COPD has been estimated at 7.6% using data from a systematic review including 28 countries [4]. However, this is likely a conservative estimate, due to under-reporting and under-diagnosis. The prevalence and burden of COPD is rising due the greater proportion of elderly people in the population [1]. It is estimated that COPD will be the third-leading cause of death by 2020 [5]. The treatment of COPD usually involves reducing exposure (e.g., smoking cessation, occupation modifications), increasing exercise, and implementing appropriate pharmacologic therapy [1]. The most common drug classes are beta2-agonists, anticholinergics, and methylxanthines. Inhaled corticosteroids (ICS) and systemic corticosteroids are often useful for acute exacerbations. -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -
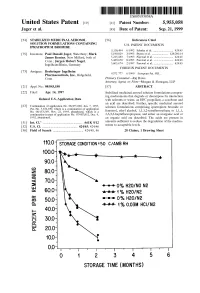
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int.