Download Product Insert (PDF)
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Dosing Time Matters
bioRxiv preprint doi: https://doi.org/10.1101/570119; this version posted March 21, 2019. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Dosing Time Matters 1 2,3 4,5,6 1* Marc D. Ruben , David F. Smith , Garret A. FitzGerald , and John B. Hogenesch 1 Division of Human Genetics, Center for Chronobiology, Department of Pediatrics, Cincinnati Children's Hospital Medical Center, 240 Albert Sabin Way, Cincinnati, OH, 45229 2 Divisions of Pediatric Otolaryngology and Pulmonary and Sleep Medicine, Cincinnati Children’s Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229 3 Department of Otolaryngology-Head and Neck Surgery, University of Cincinnati School of Medicine, 231 Albert Sabin Way, Cincinnati, OH, 45267 4 Department of Systems Pharmacology and Translational Therapeutics, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 5 Department of Medicine, at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA 6 Institute for Translational Medicine and Therapeutics (ITMAT), at the University of Pennsylvania Perelman School of Medicine, Philadelphia, PA 19104 USA *Corresponding Author. Email: [email protected] Abstract Trainees in medicine are taught to diagnose and administer treatment as needed; time-of-day is rarely considered. Yet accumulating evidence shows that ~half of human genes and physiologic functions follow daily rhythms. Circadian medicine aims to incorporate knowledge of these rhythms to enhance diagnosis and treatment. -

2019 Year in Review: Aerosol Therapy
2019 Year in Review: Aerosol Therapy Ariel Berlinski Introduction COPD Newly Approved Drugs Asthma New Devices As-Needed Inhaled Corticosteroid/Long-Acting Bronchodilator Therapy Asthma Medication Report in Adolescents and Caregivers Cystic Fibrosis Hypertonic Saline in Cystic Fibrosis Infectivity of Cough Aerosols in Cystic Fibrosis Liposomal Amikacin for MAC Lung Disease Electronic Nicotine Delivery Systems E-Cigarette or Vaping Associated Lung Injury Secondhand Exposure Summary Relevant publications related to medicinal and toxic aerosols are discussed in this review. Treatment of COPD includes a combination of long-acting bronchodilators and long-acting muscarinic antagonists. A combination of aclidinium bromide and formoterol fumarate was approved in the United States. The combination was superior to its components alone, as well as tiotropium and a salmeterol-fluticasone combination. Increased risk of an asthma exacerba- tion was reported in children exposed to electronic nicotine delivery systems. A smart inhaler capable of recording inspiratory flow was approved in the United States. The use of as-needed budesonide-formoterol was reported to be superior to scheduled budesonide and as-needed ter- butaline for the treatment of adults with mild-to-moderate asthma. A survey among teens with asthma and their caregivers revealed a disagreement in the number of inhaled controller medi- cations the teen was taking. Treatment with inhaled hypertonic saline resulted in a decreased lung clearance index in infants and preschool children with cystic fibrosis. Surgical masks were well tolerated and significantly decreased the burden of aerosolized bacteria generated by coughing in adults with cystic fibrosis. Inhaled liposomal amikacin in addition to guideline- based therapy was reported to be superior to guideline-based therapy alone in achieving nega- tive sputum cultures in adult subjects with Mycobacterium avium complex pulmonary disease. -

Terbutaline Sulfate Injection, USP
Terbutaline Sulfate Injection, USP 1 mg per mL | NDC 70860-801-01 ATHENEX AccuraSEESM PACKAGING AND LABELING BIG, BOLD AND BRIGHT — TO HELP YOU SEE IT, SAY IT AND PICK IT RIGHT DIFFERENTIATION IN EVERY LABEL, DESIGNED TO HELP REDUCE MEDICATION ERRORS PLEASE SEE FULL PRESCRIBING INFORMATION, INCLUDING BOXED WARNING, FOR TERBUTALINE SULFATE INJECTION, USP, ENCLOSED. THE NEXT GENERATION OF PHARMACY INNOVATION To order, call 1-855-273-0154 or visit www.Athenexpharma.com Terbutaline Sulfate Injection, USP 1 mg NDC 70860-801-01 1 mg per mL DESCRIPTION Glass Vial CONCENTRATION 1 mg per mL CLOSURE 13 mm UNIT OF SALE 10 vials BAR CODED Yes CHOOSE AccuraSEESM FOR YOUR PHARMACY Our proprietary, differentiated and highly-visible label designs can assist pharmacists in accurate medication selection. With a unique AccuraSEE label design for every Athenex product, we’re helping your pharmacy to reduce the risk of medication errors. The idea is simple: “So what you see is exactly what you get.” Athenex, AccuraSEE and all label designs are copyright of Athenex. ©2019 Athenex. APD-0022-02-4/19 To order, call 1-855-273-0154 or visit www.Athenexpharma.com TERBUTALINE SULFATE Injection, USP • The use of beta-adrenergic agonist bronchodilators • Terbutaline sulfate should be used during nursing alone may not be adequate to control asthma in only if the potential benefit justifies the possible risk INDICATIONS AND USAGE many patients. Early consideration should be given to to the newborn. • Terbutaline sulfate injection is indicated for the adding anti-inflammatory agents, e.g., corticosteroids. prevention and reversal of bronchospasm in patients ADVERSE REACTIONS 12 years of age and older with asthma and reversible • Terbutaline sulfate should be used with caution • Common adverse reactions reported with terbutaline bronchospasm associated with bronchitis and emphysema. -

COVID-19 Evidence Bulletin 8
COVID-19 Evidence Bulletin 8 Public Health England PHE International Epidemiology Daily Evidence Digest – 22nd April 2020 – 21st April 2020 – 20th April 2020 NICE COVID-19 rapid guideline: acute myocardial injury [NG171] Published 23rd April The purpose of this guideline is to help healthcare professionals who are not cardiology specialists identify and treat acute myocardial injury and its cardiac complications in adults with known or suspected COVID-19 but without known pre-existing cardiovascular disease. COVID-19 rapid guideline: gastrointestinal and liver conditions treated with drugs affecting the immune response [NG172] Published 23rd April The purpose of this guideline is to maximise the safety of children and adults who have gastrointestinal or liver conditions treated with drugs affecting the immune response during the COVID 19 pandemic. It also aims to protect staff from infection and enable services to make the best use of NHS resources. COVID-19 rapid guideline: managing symptoms (including at the end of life) in the community Published 3rd April, Last updated 22nd April NHS England Specialty Guides: Clinical guide for acute kidney injury in hospitalised patients with COVID-19 outside the intensive care unit during the coronavirus pandemic (22nd April - updated) Management of palliative care in hospital during the coronavirus pandemic (22nd April – updated) Department of Health and Social Care Medicines that cannot be parallel exported from the UK (22nd April) 33 medicines have been added to the parallel export list and the -

Diagnosis and Management of Asthma in Older Adults Sanjay Haresh Chotirmall, MD, Michael Watts, MD, Peter Branagan, MD, Ciaran F
PROGRESS IN GERIATRICS Diagnosis and Management of Asthma in Older Adults Sanjay Haresh Chotirmall, MD, Michael Watts, MD, Peter Branagan, MD, Ciaran F. Donegan, MD, Allan Moore, MD, and Noel Gerard McElvaney, MD Despite comprehensive guidelines established by the Euro- from 6.5% to 17.0%.5 Death rates associated with asthma pean Global Initiative for Asthma and the U.S. National depend on patient age; in a group of patients aged 55 to 59, Asthma Education and Prevention Program on the diagno- the death rate was 2.8 per 100,000 people, whereas in sis and management of asthma, its mortality in older adults people aged 60 to 64, it was 4.2 per 100, 000.6 Diagnostic continues to rise. Diagnostic and therapeutic problems and therapeutic problems contribute to many patients being contribute to older patients being inadequately treated. The inadequately treated. Despite its importance in older pa- diagnosis of asthma rests on the history and characteristic tients, asthma is particularly difficult to diagnose in this age pulmonary function testing (PFT) with the demonstration group. Symptoms typical of asthma such as intermittent of reversible airway obstruction, but there are unique prob- wheezing, breathlessness, and cough can also indicate other lems in performing this test in older patients and in its in- respiratory problems in older patients, particularly chronic terpretation. This review aims to address the difficulties in obstructive pulmonary disease (COPD). Similarly, other performing and interpreting PFT in older patients because symptoms of asthma such as chest pain or tightness may of the effects of age-related changes in lung function on be due to nonpulmonary disease such as ischemic heart respiratory physiology. -

Emea/666243/2009
European Medicines Agency London, 29 October 2009 EMEA/666243/2009 ISSUE NUMBER: 0910 MONTHLY REPORT PHARMACOVIGILANCE WORKING PARTY (PHVWP) OCTOBER 2009 PLENARY MEETING The CHMP Pharmacovigilance Working Party (PhVWP) held its October 2009 plenary meeting on 19-21 October 2009. PhVWP DISCUSSIONS ON SAFETY CONCERNS Below is a summary of the discussions regarding non-centrally authorised medicinal products in accordance with the PhVWP publication policy (see under http://www.emea.europa.eu/htms/human/phv/reports.htm). Positions agreed by the PhVWP for non- centrally authorised products are recommendations to Member States. For safety updates concerning centrally authorised products and products subject to ongoing CHMP procedures, readers are referred to the CHMP Monthly Report (see under http://www.emea.europa.eu/pressoffice/presshome.htm). The PhVWP provides advice on these products to the Committee of Medicinal Products for Human Use (CHMP) upon its request. Antipsychotics - risk of venous thromboembolism (VTE) Identify risk factors for VTE for preventive action before and during treatment with antipsychotics The PhVWP completed their review on the risk of VTE of antipsychotics1. The review was triggered by and based on data from the UK spontaneous adverse drug reactions reporting system and the published literature. The PhVWP carefully considered the data, including the limitations of both information sources, such as the lack of randomised controlled trial data, the heterogeneity of published studies and the potential confounding factors such as sedation and weight gain, commonly present in antipsychotic users. The PhVWP concluded that an association between VTE and antipsychotics cannot be excluded. Distinguishing different risk levels between the various active substances was not possible. -

Lamas for COPD Systematic Review
Comparative safety and effectiveness of inhaled long -acting agents (corticosteroids, beta agonists, anticholinergics) for chronic obstructive pulmonary disease Comprehensive Research Plan: Systematic Review Unit April 4th, 2014 Andrea C. Tricco, PhD1 and Sharon E. Straus, MD, MSc1,2 30 Bond Street, Toronto ON, M5B 1W8 www.odprn.ca [email protected] Background Chronic obstructive pulmonary disease (COPD) is characterized by airflow limitation in the lungs [1]. COPD is commonly assessed by clinical examination and spirometry. Important indicators considered in the diagnosis of COPD include age over 40 years and any of the following: 1) progressive and persistent dyspnea that worsens with exercise, 2) chronic cough, 3) chronic sputum production, 4) history of exposure to smoke from tobacco or cooking, occupational dusts and chemicals, and 5) family history of COPD [1]. COPD causes significant burden of illness, reduced quality of life, and premature death [2]. Symptoms include chronic cough, sputum production, and dyspnea [3]. The global prevalence of COPD has been estimated at 7.6% using data from a systematic review including 28 countries [4]. However, this is likely a conservative estimate, due to under-reporting and under-diagnosis. The prevalence and burden of COPD is rising due the greater proportion of elderly people in the population [1]. It is estimated that COPD will be the third-leading cause of death by 2020 [5]. The treatment of COPD usually involves reducing exposure (e.g., smoking cessation, occupation modifications), increasing exercise, and implementing appropriate pharmacologic therapy [1]. The most common drug classes are beta2-agonists, anticholinergics, and methylxanthines. Inhaled corticosteroids (ICS) and systemic corticosteroids are often useful for acute exacerbations. -

Tocolytic Therapy a Meta-Analysis and Decision Analysis
Tocolytic Therapy A Meta-Analysis and Decision Analysis David M. Haas, MD, MS, Thomas F. Imperiale, MD, Page R. Kirkpatrick, Robert W. Klein, Terrell W. Zollinger, DrPH, and Alan M. Golichowski, MD, PhD OBJECTIVE: To determine the optimal first-line tocolytic ing prostaglandin inhibitors, only 80 would deliver within agent for treatment of premature labor. 48 hours, compared with 182 for the next-best treatment. METHODS: We performed a quantitative analysis of ran- CONCLUSION: Although all current tocolytic agents domized controlled trials of tocolysis, extracting data on were superior to no treatment at delaying delivery for maternal and neonatal outcomes, and pooling rates for both 48 hours and 7 days, prostaglandin inhibitors were each outcome across trials by treatment. Outcomes were superior to the other agents and may be considered the delay of delivery for 48 hours, 7 days, and until 37 weeks; optimal first-line agent before 32 weeks of gestation to adverse effects causing discontinuation of therapy; absence delay delivery. of respiratory distress syndrome; and neonatal survival. We (Obstet Gynecol 2009;113:585–94) used weighted proportions from a random-effects meta- analysis in a decision model to determine the optimal first-line tocolytic therapy. Sensitivity analysis was per- reterm birth, defined as any birth before the gesta- formed using the standard errors of the weighted propor- Ptional age of 37 weeks, is responsible for most of the 1–3 tions. neonatal morbidity and mortality in the United States and consumes 35% of all U.S. healthcare spending on RESULTS: Fifty-eight studies satisfied the inclusion crite- 4 ria. -
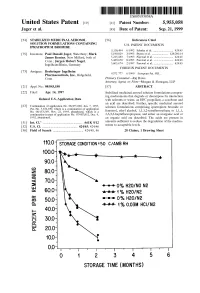
100 Storage Condition=50 C/Ambrh
USOO595.5058A United States Patent (19) 11 Patent Number: S.9SS,0589 9 Jager et al. (45) Date of Patent: Sep. 21,9 1999 54). STABILIZED MEDICINAL AEROSOL 56) References Cited SOLUTION FORMULATIONS CONTAINING U.S. PATENT DOCUMENTS IPRATROPIUM BROMIDE a 5,118,494 6/1992 Schultz et al. ............................ 424/45 75 Inventors: Paul Donald Jager, Waterbury; Mark 5,190,029 3/1993 Byron et al. ... ... 128/200.14 James Kontny, New Milford, both of 5,225,183 7/1993 Purewal et al. ........................... 424/45 Conn.; Jurgen Hubert Nagel 5.439,670 8/1995 Purewal et al. ... 424/45 Ingelheim/Rhein, Germany s 5,605,674 2/1997 Purewal et al. ........................... 424/45 FOREIGN PATENT DOCUMENTS 73 Assignee: Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, 0372 777 6/1990 European Pat. Off.. Conn. Primary Examiner Raj Bawa Attorney, Agent, or Firm Morgan & Finnegan, LLP 21 Appl.pp No.: 08/843,180 57 ABSTRACT 22 Filed: Apr. 14, 1997 Stabilized medicinal aeroSol Solution formulations compris O O ing medicaments that degrade or decompose by interaction Related U.S. Application Data with solvents or water, an HFC propellant, a cosolvent and an acid are described. Further, Specific medicinal aeroSol 63 Staggypt.NE "A iGs Solution formulations comprising ipratropium bromide or No. 08/153.549, Nov. 22, 1993, abandoned E. is a fenoterol, ethyl alcohol, 1,1,1,2-tetrafluoroethane or 1,1,1, continuation-in-part of application No. 07/987,852, Dec. 9, 2,3,3,3-heptafluoropropane, and either an inorganic acid or 1992, abandoned. an organic acid are described. The acids are present in 51 Int. -

210428Orig1s000
CENTER FOR DRUG EVALUATION AND RESEARCH APPLICATION NUMBER: 210428Orig1s000 OTHER REVIEW(S) DIVISION OF CARDIOVASCULAR AND RENAL PRODUCTS Regulatory Project Manager Overview I. GENERAL INFORMATION NDA: 210428 Drug: Metoprolol Succinate Extended-Release Capsules, 25 mg, 50 mg, 100 mg and 200 mg Class: Beta- Blocker Applicant: Sun Pharmaceutical Industries Limited Proposed Indications: Hypertension, Angina Pectoris & Heart Failure Date of submission: March 30, 2017 PDUFA date: January 30, 2018 II. REVIEW TEAM Office of New Drugs, Office of Drug Evaluation I: Division of Cardiovascular & Renal Product Norman Stockbridge, MD, PhD, Director Mary Ross Southworth, PharmD, Deputy Director for Safety Michael Monteleone, MS, RAC, Assistant Director for Labeling Martina Sahre, PhD, Cross Discipline Team Leader Fortunato Senatore, MD, Clinical Reviewer Albert DeFelice, PhD, Non-Clinical Supervisor Muriel Saulnier, PhD, Non-Clinical Reviewer Edward Fromm, RPh, RAC, Chief, Project Management Staff Maryam Changi, PharmD, Regulatory Project Manager Office of Pharmaceutical Quality: Wendy Wilson-Lee, PhD, Application Technical Lead Grafton Adams, Regulatory Business Process Manager Milton Sloan, PhD, Drug Product Reviewer Rohit Tiwari, PhD, Drug Substance Reviewer Ben Stevens, PhD, Drug Substance Team Leader Kaushal Dave, PhD, Biopharmaceutical Reviewer Wu, Ta-Chin, PhD, Biopharmaceutical Team Leader Viviana Matta, PhD, Facility Reviewer Ruth Moore, PhD, Facility Team Leader Chunsheng Cai, PhD, Process Reviewer NDA 210428 RPM review Page 1 Reference ID: 42132034213643 Labeling/PMC-PMR to Sponsor: November 30, 2017 PDUFA Date: January 30, 2018 (Standard, 10-Month) PDUFA Goal Date: January 30, 2018 Approval letter: January 26, 2018 7. Reviews a) Divisional Memorandum: (January 26, 2018) Dr. Stockbridge indicated his concurrence on Dr. Sahre’s CDTL memo. -

Pharmacology and Therapeutics of Bronchodilators
1521-0081/12/6403-450–504$25.00 PHARMACOLOGICAL REVIEWS Vol. 64, No. 3 Copyright © 2012 by The American Society for Pharmacology and Experimental Therapeutics 4580/3762238 Pharmacol Rev 64:450–504, 2012 ASSOCIATE EDITOR: DAVID R. SIBLEY Pharmacology and Therapeutics of Bronchodilators Mario Cazzola, Clive P. Page, Luigino Calzetta, and M. Gabriella Matera Department of Internal Medicine, Unit of Respiratory Clinical Pharmacology, University of Rome ‘Tor Vergata,’ Rome, Italy (M.C., L.C.); Department of Pulmonary Rehabilitation, San Raffaele Pisana Hospital, Istituto di Ricovero e Cura a Carattere Scientifico, Rome, Italy (M.C., L.C.); Sackler Institute of Pulmonary Pharmacology, Institute of Pharmaceutical Science, King’s College London, London, UK (C.P.P., L.C.); and Department of Experimental Medicine, Unit of Pharmacology, Second University of Naples, Naples, Italy (M.G.M.) Abstract............................................................................... 451 I. Introduction: the physiological rationale for using bronchodilators .......................... 452 II. -Adrenergic receptor agonists .......................................................... 455 A. A history of the development of -adrenergic receptor agonists: from nonselective  Downloaded from adrenergic receptor agonists to 2-adrenergic receptor-selective drugs.................... 455  B. Short-acting 2-adrenergic receptor agonists........................................... 457 1. Albuterol........................................................................ 457 -

Chronic Obstructiv Chronic Obstructive Pulmonary Disease
pat hways Chronic obstructive pulmonary disease: beclometasone, formoterol and glycopyrronium (Trimbow) Evidence summary Published: 3 May 2018 nice.org.uk/guidance/es17 Key points The content of this evidence summary was up-to-date in May 2018. See summaries of product characteristics (SPCs), British national formulary (BNF) or the Medicines and Healthcare products Regulatory Agency (MHRA) or NICE websites for up-to-date information. Regulatory status: Beclometasone/formoterol/glycopyrronium (Trimbow, Chiesi Limited) received a European marketing authorisation in July 2017. This triple-therapy inhaler contains an inhaled corticosteroid (ICS), long-acting beta-2 agonist (LABA) and long-acting muscarinic antagonist (LAMA). It is licensed for maintenance treatment of adults with moderate-to-severe chronic obstructive pulmonary disease (COPD) who are not adequately treated by a combination of an ICS and a LABA. Overview This evidence summary discusses 3 randomised controlled trials (TRILOGY, TRINITY and TRIBUTE) looking at the safety and efficacy of beclometasone/formoterol/glycopyrronium in people with COPD with severe or very severe airflow limitation, symptoms despite treatment and a history of exacerbations. © NICE 2018. All rights reserved. Subject to Notice of rights (https://www.nice.org.uk/terms-and- Page 1 of conditions#notice-of-rights). 78 Chronic obstructive pulmonary disease: beclometasone, formoterol and glycopyrronium (Trimbow) (ES17) Overall, the studies found small, statistically significant improvements in lung function, rates of moderate-to-severe exacerbations of COPD and health-related quality-of-life scores with beclometasone/formoterol/glycopyrronium compared with beclometasone/formoterol or indacaterol/glycopyrronium dual therapy, or tiotropium alone. The improvements may be of limited clinical importance. In TRILOGY and TRINITY, improvements in primary outcomes relating to lung function and exacerbation rates just reached the level considered to be clinically important.