Succession of Diptera on Dead Beech Wood: a 10-Year Study
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Topic Paper Chilterns Beechwoods
. O O o . 0 O . 0 . O Shoping growth in Docorum Appendices for Topic Paper for the Chilterns Beechwoods SAC A summary/overview of available evidence BOROUGH Dacorum Local Plan (2020-2038) Emerging Strategy for Growth COUNCIL November 2020 Appendices Natural England reports 5 Chilterns Beechwoods Special Area of Conservation 6 Appendix 1: Citation for Chilterns Beechwoods Special Area of Conservation (SAC) 7 Appendix 2: Chilterns Beechwoods SAC Features Matrix 9 Appendix 3: European Site Conservation Objectives for Chilterns Beechwoods Special Area of Conservation Site Code: UK0012724 11 Appendix 4: Site Improvement Plan for Chilterns Beechwoods SAC, 2015 13 Ashridge Commons and Woods SSSI 27 Appendix 5: Ashridge Commons and Woods SSSI citation 28 Appendix 6: Condition summary from Natural England’s website for Ashridge Commons and Woods SSSI 31 Appendix 7: Condition Assessment from Natural England’s website for Ashridge Commons and Woods SSSI 33 Appendix 8: Operations likely to damage the special interest features at Ashridge Commons and Woods, SSSI, Hertfordshire/Buckinghamshire 38 Appendix 9: Views About Management: A statement of English Nature’s views about the management of Ashridge Commons and Woods Site of Special Scientific Interest (SSSI), 2003 40 Tring Woodlands SSSI 44 Appendix 10: Tring Woodlands SSSI citation 45 Appendix 11: Condition summary from Natural England’s website for Tring Woodlands SSSI 48 Appendix 12: Condition Assessment from Natural England’s website for Tring Woodlands SSSI 51 Appendix 13: Operations likely to damage the special interest features at Tring Woodlands SSSI 53 Appendix 14: Views About Management: A statement of English Nature’s views about the management of Tring Woodlands Site of Special Scientific Interest (SSSI), 2003. -
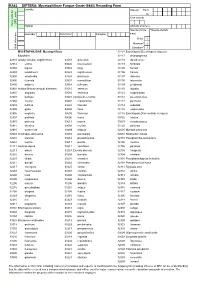
Ra82 Diptera
RA82 DIPTERA: Mycetophilinae Fungus Gnats (6480) Recording Form Locality Date(s) from: to: Vice county GPS users Grey cells for Habitat Altitude (metres) Source (circle *Source details Recorder Determiner Compiler one) Field 1 Museum* 2 Grid reference Literature* 3 MYCETOPHILIDAE: Mycetophilinae 33101 Exechiopsis (Exechiopsis) clypeata Exechiini 33117 dryaspagensis 32801 Allodia (Allodia) anglofennica 33519 griseolum33118 dumitrescae 32912 embla 33526 intermedium33119 fimbriata 32802 lugens 33522 kingi33120 furcata 32803 lundstroemi 33523 nigrofuscum33106 hammi 32804 ornaticollis 33524 proximum33107 indecisa 32806 truncata 33527 rosmellitum33108 intersecta 32805 zaitzevi 33514 ruficorne33109 jenkinsoni 32901 Allodia (Brachycampta) alternans 33515 serenum33110 ligulata 32910 angulata 33516 sericoma33121 magnicauda 32903 barbata 33601 Cordyla brevicornis33112 pseudindecisa 32904 czernyi 33602 crassicornis33113 pulchella 32909 foliifera 33603 fasciata33114 subulata 32905 grata 33604 fissa33115 unguiculata 32906 neglecta 33605 flaviceps33116 Exechiopsis (Xenexechia) crucigera 32907 pistillata 33606 fusca33002 leptura 32915 protenta 33613 insons33003 membranacea 32914 silvatica 33608 murina33122 pollicata 32916 westerholti 33609 nitidula32605 Myrosia maculosa 32602 Allodiopsis domestica 33610 parvipalpis32601 Notolopha cristata 32610 korolevi 33614 pseudomurina32701 Pseudexechia aurivernica 32607 rustica 33611 pusilla32706 monica 32212 Anatella alpina 33612 semiflava32705 parallela 32213 ankeli 33201 Exechia bicincta32703 trisignata 32216 bremia -

R. P. LANE (Department of Entomology), British Museum (Natural History), London SW7 the Diptera of Lundy Have Been Poorly Studied in the Past
Swallow 3 Spotted Flytcatcher 28 *Jackdaw I Pied Flycatcher 5 Blue Tit I Dunnock 2 Wren 2 Meadow Pipit 10 Song Thrush 7 Pied Wagtail 4 Redwing 4 Woodchat Shrike 1 Blackbird 60 Red-backed Shrike 1 Stonechat 2 Starling 15 Redstart 7 Greenfinch 5 Black Redstart I Goldfinch 1 Robin I9 Linnet 8 Grasshopper Warbler 2 Chaffinch 47 Reed Warbler 1 House Sparrow 16 Sedge Warbler 14 *Jackdaw is new to the Lundy ringing list. RECOVERIES OF RINGED BIRDS Guillemot GM I9384 ringed 5.6.67 adult found dead Eastbourne 4.12.76. Guillemot GP 95566 ringed 29.6.73 pullus found dead Woolacombe, Devon 8.6.77 Starling XA 92903 ringed 20.8.76 found dead Werl, West Holtun, West Germany 7.10.77 Willow Warbler 836473 ringed 14.4.77 controlled Portland, Dorset 19.8.77 Linnet KC09559 ringed 20.9.76 controlled St Agnes, Scilly 20.4.77 RINGED STRANGERS ON LUNDY Manx Shearwater F.S 92490 ringed 4.9.74 pullus Skokholm, dead Lundy s. Light 13.5.77 Blackbird 3250.062 ringed 8.9.75 FG Eksel, Belgium, dead Lundy 16.1.77 Willow Warbler 993.086 ringed 19.4.76 adult Calf of Man controlled Lundy 6.4.77 THE DIPTERA (TWO-WINGED FLffiS) OF LUNDY ISLAND R. P. LANE (Department of Entomology), British Museum (Natural History), London SW7 The Diptera of Lundy have been poorly studied in the past. Therefore, it is hoped that the production of an annotated checklist, giving an indication of the habits and general distribution of the species recorded will encourage other entomologists to take an interest in the Diptera of Lundy. -

Dipterists Forum
BULLETIN OF THE Dipterists Forum Bulletin No. 76 Autumn 2013 Affiliated to the British Entomological and Natural History Society Bulletin No. 76 Autumn 2013 ISSN 1358-5029 Editorial panel Bulletin Editor Darwyn Sumner Assistant Editor Judy Webb Dipterists Forum Officers Chairman Martin Drake Vice Chairman Stuart Ball Secretary John Kramer Meetings Treasurer Howard Bentley Please use the Booking Form included in this Bulletin or downloaded from our Membership Sec. John Showers website Field Meetings Sec. Roger Morris Field Meetings Indoor Meetings Sec. Duncan Sivell Roger Morris 7 Vine Street, Stamford, Lincolnshire PE9 1QE Publicity Officer Erica McAlister [email protected] Conservation Officer Rob Wolton Workshops & Indoor Meetings Organiser Duncan Sivell Ordinary Members Natural History Museum, Cromwell Road, London, SW7 5BD [email protected] Chris Spilling, Malcolm Smart, Mick Parker Nathan Medd, John Ismay, vacancy Bulletin contributions Unelected Members Please refer to guide notes in this Bulletin for details of how to contribute and send your material to both of the following: Dipterists Digest Editor Peter Chandler Dipterists Bulletin Editor Darwyn Sumner Secretary 122, Link Road, Anstey, Charnwood, Leicestershire LE7 7BX. John Kramer Tel. 0116 212 5075 31 Ash Tree Road, Oadby, Leicester, Leicestershire, LE2 5TE. [email protected] [email protected] Assistant Editor Treasurer Judy Webb Howard Bentley 2 Dorchester Court, Blenheim Road, Kidlington, Oxon. OX5 2JT. 37, Biddenden Close, Bearsted, Maidstone, Kent. ME15 8JP Tel. 01865 377487 Tel. 01622 739452 [email protected] [email protected] Conservation Dipterists Digest contributions Robert Wolton Locks Park Farm, Hatherleigh, Oakhampton, Devon EX20 3LZ Dipterists Digest Editor Tel. -

Millichope Park and Estate Invertebrate Survey 2020
Millichope Park and Estate Invertebrate survey 2020 (Coleoptera, Diptera and Aculeate Hymenoptera) Nigel Jones & Dr. Caroline Uff Shropshire Entomology Services CONTENTS Summary 3 Introduction ……………………………………………………….. 3 Methodology …………………………………………………….. 4 Results ………………………………………………………………. 5 Coleoptera – Beeetles 5 Method ……………………………………………………………. 6 Results ……………………………………………………………. 6 Analysis of saproxylic Coleoptera ……………………. 7 Conclusion ………………………………………………………. 8 Diptera and aculeate Hymenoptera – true flies, bees, wasps ants 8 Diptera 8 Method …………………………………………………………… 9 Results ……………………………………………………………. 9 Aculeate Hymenoptera 9 Method …………………………………………………………… 9 Results …………………………………………………………….. 9 Analysis of Diptera and aculeate Hymenoptera … 10 Conclusion Diptera and aculeate Hymenoptera .. 11 Other species ……………………………………………………. 12 Wetland fauna ………………………………………………….. 12 Table 2 Key Coleoptera species ………………………… 13 Table 3 Key Diptera species ……………………………… 18 Table 4 Key aculeate Hymenoptera species ……… 21 Bibliography and references 22 Appendix 1 Conservation designations …………….. 24 Appendix 2 ………………………………………………………… 25 2 SUMMARY During 2020, 811 invertebrate species (mainly beetles, true-flies, bees, wasps and ants) were recorded from Millichope Park and a small area of adjoining arable estate. The park’s saproxylic beetle fauna, associated with dead wood and veteran trees, can be considered as nationally important. True flies associated with decaying wood add further significant species to the site’s saproxylic fauna. There is also a strong -

Diversity and Resource Choice of Flower-Visiting Insects in Relation to Pollen Nutritional Quality and Land Use
Diversity and resource choice of flower-visiting insects in relation to pollen nutritional quality and land use Diversität und Ressourcennutzung Blüten besuchender Insekten in Abhängigkeit von Pollenqualität und Landnutzung Vom Fachbereich Biologie der Technischen Universität Darmstadt zur Erlangung des akademischen Grades eines Doctor rerum naturalium genehmigte Dissertation von Dipl. Biologin Christiane Natalie Weiner aus Köln Berichterstatter (1. Referent): Prof. Dr. Nico Blüthgen Mitberichterstatter (2. Referent): Prof. Dr. Andreas Jürgens Tag der Einreichung: 26.02.2016 Tag der mündlichen Prüfung: 29.04.2016 Darmstadt 2016 D17 2 Ehrenwörtliche Erklärung Ich erkläre hiermit ehrenwörtlich, dass ich die vorliegende Arbeit entsprechend den Regeln guter wissenschaftlicher Praxis selbständig und ohne unzulässige Hilfe Dritter angefertigt habe. Sämtliche aus fremden Quellen direkt oder indirekt übernommene Gedanken sowie sämtliche von Anderen direkt oder indirekt übernommene Daten, Techniken und Materialien sind als solche kenntlich gemacht. Die Arbeit wurde bisher keiner anderen Hochschule zu Prüfungszwecken eingereicht. Osterholz-Scharmbeck, den 24.02.2016 3 4 My doctoral thesis is based on the following manuscripts: Weiner, C.N., Werner, M., Linsenmair, K.-E., Blüthgen, N. (2011): Land-use intensity in grasslands: changes in biodiversity, species composition and specialization in flower-visitor networks. Basic and Applied Ecology 12 (4), 292-299. Weiner, C.N., Werner, M., Linsenmair, K.-E., Blüthgen, N. (2014): Land-use impacts on plant-pollinator networks: interaction strength and specialization predict pollinator declines. Ecology 95, 466–474. Weiner, C.N., Werner, M , Blüthgen, N. (in prep.): Land-use intensification triggers diversity loss in pollination networks: Regional distinctions between three different German bioregions Weiner, C.N., Hilpert, A., Werner, M., Linsenmair, K.-E., Blüthgen, N. -

Recent Noteworthy Findings of Fungus Gnats from Finland and Northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae)
Biodiversity Data Journal 2: e1068 doi: 10.3897/BDJ.2.e1068 Taxonomic paper Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae) Jevgeni Jakovlev†, Jukka Salmela ‡,§, Alexei Polevoi|, Jouni Penttinen ¶, Noora-Annukka Vartija# † Finnish Environment Insitutute, Helsinki, Finland ‡ Metsähallitus (Natural Heritage Services), Rovaniemi, Finland § Zoological Museum, University of Turku, Turku, Finland | Forest Research Institute KarRC RAS, Petrozavodsk, Russia ¶ Metsähallitus (Natural Heritage Services), Jyväskylä, Finland # Toivakka, Myllyntie, Finland Corresponding author: Jukka Salmela ([email protected]) Academic editor: Vladimir Blagoderov Received: 10 Feb 2014 | Accepted: 01 Apr 2014 | Published: 02 Apr 2014 Citation: Jakovlev J, Salmela J, Polevoi A, Penttinen J, Vartija N (2014) Recent noteworthy findings of fungus gnats from Finland and northwestern Russia (Diptera: Ditomyiidae, Keroplatidae, Bolitophilidae and Mycetophilidae). Biodiversity Data Journal 2: e1068. doi: 10.3897/BDJ.2.e1068 Abstract New faunistic data on fungus gnats (Diptera: Sciaroidea excluding Sciaridae) from Finland and NW Russia (Karelia and Murmansk Region) are presented. A total of 64 and 34 species are reported for the first time form Finland and Russian Karelia, respectively. Nine of the species are also new for the European fauna: Mycomya shewelli Väisänen, 1984,M. thula Väisänen, 1984, Acnemia trifida Zaitzev, 1982, Coelosia gracilis Johannsen, 1912, Orfelia krivosheinae Zaitzev, 1994, Mycetophila biformis Maximova, 2002, M. monstera Maximova, 2002, M. uschaica Subbotina & Maximova, 2011 and Trichonta palustris Maximova, 2002. Keywords Sciaroidea, Fennoscandia, faunistics © Jakovlev J et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 .................................. -

Bilimsel Araştırma Projesi (8.011Mb)
1 T.C. GAZİOSMANPAŞA ÜNİVERSİTESİ Bilimsel Araştırma Projeleri Komisyonu Sonuç Raporu Proje No: 2008/26 Projenin Başlığı AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Proje Yöneticisi Prof.Dr. Kenan KARA Bitki Koruma Anabilim Dalı Araştırmacı Turgut ATAY Bitki Koruma Anabilim Dalı (Kasım / 2011) 2 T.C. GAZİOSMANPAŞA ÜNİVERSİTESİ Bilimsel Araştırma Projeleri Komisyonu Sonuç Raporu Proje No: 2008/26 Projenin Başlığı AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Proje Yöneticisi Prof.Dr. Kenan KARA Bitki Koruma Anabilim Dalı Araştırmacı Turgut ATAY Bitki Koruma Anabilim Dalı (Kasım / 2011) ÖZET* 3 AMASYA, SİVAS VE TOKAT İLLERİNİN KELKİT HAVZASINDAKİ FARKLI BÖCEK TAKIMLARINDA BULUNAN TACHINIDAE (DIPTERA) TÜRLERİ ÜZERİNDE ÇALIŞMALAR Yapılan bu çalışma ile Amasya, Sivas ve Tokat illerinin Kelkit havzasına ait kısımlarında bulunan ve farklı böcek takımlarında parazitoit olarak yaşayan Tachinidae (Diptera) türleri, bunların tanımları ve yayılışlarının ortaya konulması amaçlanmıştır. Bunun için farklı böcek takımlarına ait türler laboratuvarda kültüre alınarak parazitoit olarak yaşayan Tachinidae türleri elde edilmiştir. Kültüre alınan Lepidoptera takımına ait türler içerisinden, Euproctis chrysorrhoea (L.), Lymantria dispar (L.), Malacosoma neustrium (L.), Smyra dentinosa Freyer, Thaumetopoea solitaria Freyer, Thaumetopoea sp. ve Vanessa sp.,'den parazitoit elde edilmiş, -

The Diptera of Lancashire and Cheshire: Craneflies and Winter Gnats
The Diptera of Lancashire and Cheshire: Craneflies and Winter Gnats by Phil Brighton 32, Wadeson Way, Croft, Warrington WA3 7JS [email protected] Version 1.1 26 November 2017 1 Summary This document provides a new checklist for the craneflies and winter gnats (Tipuloidea, Ptychopteridae and Trichoceridae) to extend the lists of the diptera of Lancashire and Cheshire first published by Kidd and Bindle in 1959. Overall statistics on recording activity are given by decade and hectad. Checklists are presented for each of the three Watsonian vice-counties 58, 59, and 60 detailing for each species the number of records, year of earliest and most recent record, and the number of hectads with records. A combined checklist showing distribution by the three vice-counties is also included, covering a total of 264 species, amounting to 75% of the current British checklist. Introduction This report is the third in a series to update and extend the partial checklist of the diptera of Lancashire and Cheshire published in 1959 by Leonard Kidd and Alan Brindle1. There were two previous updates, in 19642 and 19713. The previous reports in this series cover the soldierflies and allies4 and the Sepsidae5, the latter family not having been covered in Ref 1. The reader is referred to the first two reports for the background and rationale of these checklists, as well as the history of diptera recording and available data sources. The description of methodology is also kept to a minimum in the present report: only significant differences from the previous publications will be outlined. -

Volume 2, Chapter 12-19: Terrestrial Insects: Holometabola-Diptera
Glime, J. M. 2017. Terrestrial Insects: Holometabola – Diptera Nematocera 2. In: Glime, J. M. Bryophyte Ecology. Volume 2. 12-19-1 Interactions. Ebook sponsored by Michigan Technological University and the International Association of Bryologists. eBook last updated 19 July 2020 and available at <http://digitalcommons.mtu.edu/bryophyte-ecology2/>. CHAPTER 12-19 TERRESTRIAL INSECTS: HOLOMETABOLA – DIPTERA NEMATOCERA 2 TABLE OF CONTENTS Cecidomyiidae – Gall Midges ........................................................................................................................ 12-19-2 Mycetophilidae – Fungus Gnats ..................................................................................................................... 12-19-3 Sciaridae – Dark-winged Fungus Gnats ......................................................................................................... 12-19-4 Ceratopogonidae – Biting Midges .................................................................................................................. 12-19-6 Chironomidae – Midges ................................................................................................................................. 12-19-9 Belgica .................................................................................................................................................. 12-19-14 Culicidae – Mosquitoes ................................................................................................................................ 12-19-15 Simuliidae – Blackflies -

Ad Hoc Referees Committee for This Issue Thomas Dirnböck
COMITATO DI REVISIONE PER QUESTO NUMERO – Ad hoc referees committee for this issue Thomas Dirnböck Umweltbundesamt GmbH Studien & Beratung II, Spittelauer Lände 5, 1090 Wien, Austria Marco Kovac Slovenian Forestry Institute, Vecna pot 2, 1000 Ljubljana, Slovenija Susanna Nocentini Università degli Studi di Firenze, DISTAF, Via S. Bonaventura 13, 50145 Firenze Ralf Ohlemueller Department of Biology, University of York, PO Box 373, York YO10 5YW, UK Sandro Pignatti Orto Botanico di Roma, Dipartimento di Biologia Vegetale, L.go Cristina di Svezia, 24, 00165 Roma Stergios Pirintsos Department of Biology, University of Crete, P.O.Box 2208, 71409 Heraklion, Greece Matthias Plattner Hintermann & Weber AG, Oeko-Logische Beratung Planung Forschung, Hauptstrasse 52, CH-4153 Reinach Basel Arne Pommerening School of Agricultural & Forest Sciences, University of Wales, Bangor, Gwynedd LL57 2UW, DU/ UK Roberto Scotti Università degli Studi di Sassari, DESA, Nuoro branch, Via C. Colombo 1, 08100 Nuoro Franz Starlinger Forstliche Bundesversuchsanstalt Wien, A 1131 Vienna, Austria Silvia Stofer Eidgenössische Forschungsanstalt für Wald, Schnee und Landschaft – WSL, Zürcherstrasse 111, CH-8903 Birmensdorf, Switzerland Norman Woodley Systematic Entomology Lab-USDA , c/o Smithsonian Institution NHB-168 , O Box 37012 Washington, DC 20013-7012 CURATORI DI QUESTO NUMERO – Editors Marco Ferretti, Bruno Petriccione, Gianfranco Fabbio, Filippo Bussotti EDITORE – Publisher C.R.A. - Istituto Sperimentale per la Selvicoltura Viale Santa Margherita, 80 – 52100 Arezzo Tel.. ++39 0575 353021; Fax. ++39 0575 353490; E-mail:[email protected] Volume 30, Supplemento 2 - 2006 LIST OF CONTRIBUTORS C.R.A.A - ISTITUTO N SPERIMENTALE N A PER LA LSELVICOLTURA I (in alphabetic order) Allegrini, M. C.