Cambodian Journal of Natural History
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

5.4 Insect Visitors to Marianthus Aquilonaris and Surrounding Flora
REPORT: Insect visitors to Marianthus aquilonaris and surrounding flora Nov 2-4, 2019 Kit Prendergast, Native bee scientist BSc First Class Honours, PhD researcher and Forrest Scholar On behalf of Botanica Consulting 1 REPORT: Insect visitors to Marianthus aquilonaris and surrounding flora Nov 2-4 2019 Kit Prendergast, Native bee scientist Background Marianthus aquilonaris (Fig. 1) was declared as Rare Flora under the Western Australian Wildlife Conservation Act 1950 in 2002 under the name Marianthus sp. Bremer, and is ranked as Critically Endangered (CR) under the International Union for Conservation of Nature (IUCN 2001) criteria B1ab(iii,v)+2ab(iii,v); C2a(ii) due to its extent of occurrence being less than 100 km2, its area of occupancy being less than 10 km2, a continuing decline in the area, extent and/or quality of its habitat and number of mature individuals and there being less than 250 mature individuals known at the time of ranking (Appendix A). However, it no longer meets these criteria as more plants have been found, and a recommendation has been proposed to be made by DBCA to the Threatened Species Scientific Committee (TSSC) to change its conservation status to CR B1ab(iii,v)+2ab(iii,v) (Appendix A), but this recommendation has not gone ahead (DEC, 2010). Despite its listing as CR under the Western Australian Biodiversity Conservation Act 2016, the species is not currently listed under the Environment Protection and Biodiversity Conservation Act 1999. The main threats to the species are mining/exploration, track maintenance and inappropriate fire regimes (DEC, 2010). Fig. 1. Marianthus aquilonaris, showing flower, buds and leaves. -

Invaded: a Study of Wild Pollinator Responses to Introduced Flower Species
Invaded: A Study of Wild Pollinator Responses to Introduced Flower Species UNDERC West 2019 By Eileen C Reeves Advisor: Katherine Barrett 1 Abstract Pollinators are threatened primarily by habitat loss and fragmentation. Agriculture and ranching form large monocultures and areas of primarily wind pollinated grasses. In places where flowers are found, including state parks and national wildlife refuges, invasive species thrive and, in some areas, dominate. This study aims to determine whether native pollinators show feeding preferences between invasive and native flowers. If pollinators show little to no preference, then areas with any flowers, even invasive ones, should support healthy pollinator communities. I anticipate that Lepidoptera (butterflies and moths) and Anthophila (bees) will show greater preference for native species while Diptera (flies) will not show any preference My results show no significant preference for either native or introduced species on the whole. One genus showed significant preference for introduced flowers. Time of day was the most significant factor. Introduction Invasive species pose one of the greatest threats to biodiversity today. Invasive flowers outcompete native species for water, nutrients, and sun, which often leads to shifts in plant species composition and diversity, which can impact associated insect communities (Ridenour and Callaway, 2003; Callaway and Ridenour, 2004). Invasive plant/native plant interactions are well documented, but less so plant-insect interactions. This study set out to find whether invasive and introduced plant communities can host healthy pollinator assemblages. Plant-insect interactions date back to the origin of flowers. Flowering plant diversity drastically increased as insect diversity did. Flowers co-evolved with insects due to pollination. -

Sensory and Cognitive Adaptations to Social Living in Insect Societies Tom Wenseleersa,1 and Jelle S
COMMENTARY COMMENTARY Sensory and cognitive adaptations to social living in insect societies Tom Wenseleersa,1 and Jelle S. van Zwedena A key question in evolutionary biology is to explain the solitarily or form small annual colonies, depending upon causes and consequences of the so-called “major their environment (9). And one species, Lasioglossum transitions in evolution,” which resulted in the pro- marginatum, is even known to form large perennial euso- gressive evolution of cells, organisms, and animal so- cial colonies of over 400 workers (9). By comparing data cieties (1–3). Several studies, for example, have now from over 30 Halictine bees with contrasting levels of aimed to determine which suite of adaptive changes sociality, Wittwer et al. (7) now show that, as expected, occurred following the evolution of sociality in insects social sweat bee species invest more in sensorial machin- (4). In this context, a long-standing hypothesis is that ery linked to chemical communication, as measured by the evolution of the spectacular sociality seen in in- the density of their antennal sensillae, compared with sects, such as ants, bees, or wasps, should have gone species that secondarily reverted back to a solitary life- hand in hand with the evolution of more complex style. In fact, the same pattern even held for the socially chemical communication systems, to allow them to polymorphic species L. albipes if different populations coordinate their complex social behavior (5). Indeed, with contrasting levels of sociality were compared (Fig. whereas solitary insects are known to use pheromone 1, Inset). This finding suggests that the increased reliance signals mainly in the context of mate attraction and on chemical communication that comes with a social species-recognition, social insects use chemical sig- lifestyle indeed selects for fast, matching adaptations in nals in a wide variety of contexts: to communicate their sensory systems. -

A History of the Anlong Veng Community a History Of
A HIstoRy Of Anlong Veng CommunIty A wedding in Anlong Veng in the early 1990s. (Cover photo) Aer Vietnamese forces entered Cambodia in 1979, many Khmer Rouge forces scaered to the jungles, mountains, and border areas. Mountain 1003 was a prominent Khmer Rouge military base located within the Dangrek Mountains along the Cambodian-Thai border, not far from Anlong Veng. From this military base, the Khmer Rouge re-organized and prepared for the long struggle against Vietnamese and the People’s Republic of Kampuchea government forces. Eventually, it was from this base, Khmer Rouge forces would re-conquer and sele Anlong Veng in early 1990 (and a number of other locations) until their re-integration into Cambodian society in late 1998. In many ways, life in Anlong Veng was as difficult and dangerous as it was in Mountain 1003. As one of the KR strongholds, Anlong Veng served as one of the key launching points for Khmer Rouge guerrilla operations in Cambodia, and it was subject to constant aacks by Cambodian government forces. Despite the perilous circumstances and harsh environment, the people who lived in Anlong Veng endeavored, whenever possible, to re-connect with and maintain their rich cultural heritage. Tossed from the seat of power in 1979, the Khmer Rouge were unable to sustain their rigid ideo- logical policies, particularly as it related to community and family life. During the Democratic Movement of the Khmer Rouge Final Stronghold Kampuchea regime, 1975–79, the Khmer Rouge prohibited the traditional Cambodian wedding ceremony. Weddings were arranged by Khmer Rouge leaders and cadre, who oen required mass ceremonies, with lile regard for tradition or individual distinction. -
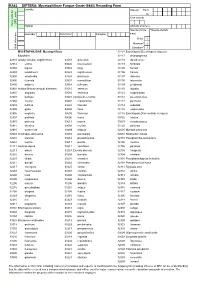
Ra82 Diptera
RA82 DIPTERA: Mycetophilinae Fungus Gnats (6480) Recording Form Locality Date(s) from: to: Vice county GPS users Grey cells for Habitat Altitude (metres) Source (circle *Source details Recorder Determiner Compiler one) Field 1 Museum* 2 Grid reference Literature* 3 MYCETOPHILIDAE: Mycetophilinae 33101 Exechiopsis (Exechiopsis) clypeata Exechiini 33117 dryaspagensis 32801 Allodia (Allodia) anglofennica 33519 griseolum33118 dumitrescae 32912 embla 33526 intermedium33119 fimbriata 32802 lugens 33522 kingi33120 furcata 32803 lundstroemi 33523 nigrofuscum33106 hammi 32804 ornaticollis 33524 proximum33107 indecisa 32806 truncata 33527 rosmellitum33108 intersecta 32805 zaitzevi 33514 ruficorne33109 jenkinsoni 32901 Allodia (Brachycampta) alternans 33515 serenum33110 ligulata 32910 angulata 33516 sericoma33121 magnicauda 32903 barbata 33601 Cordyla brevicornis33112 pseudindecisa 32904 czernyi 33602 crassicornis33113 pulchella 32909 foliifera 33603 fasciata33114 subulata 32905 grata 33604 fissa33115 unguiculata 32906 neglecta 33605 flaviceps33116 Exechiopsis (Xenexechia) crucigera 32907 pistillata 33606 fusca33002 leptura 32915 protenta 33613 insons33003 membranacea 32914 silvatica 33608 murina33122 pollicata 32916 westerholti 33609 nitidula32605 Myrosia maculosa 32602 Allodiopsis domestica 33610 parvipalpis32601 Notolopha cristata 32610 korolevi 33614 pseudomurina32701 Pseudexechia aurivernica 32607 rustica 33611 pusilla32706 monica 32212 Anatella alpina 33612 semiflava32705 parallela 32213 ankeli 33201 Exechia bicincta32703 trisignata 32216 bremia -

History a Work in Progress in One-Time KR Stronghold May Titthara January 25, 2011
History a work in progress in one-time KR stronghold May Titthara January 25, 2011 Sitting under a tree outside Malai High School, 20-year-old Phen Soeurm offers a dismissive approach to his history class typical of many his age. As the teacher lectures, “the class just listens without paying attention at all,” Phen Soeurm says. “They just want to kill time.” Here in this dusty district of Banteay Meanchey province, however, there is more to this phenomenon than a simple case of student laziness. The lecture in question covers the history of the Democratic Kampuchea regime, an understandably sensitive topic in this former Khmer Rouge stronghold. “Most students don’t want to study Khmer Rouge history because they don’t want to be reminded of what happened, and because all of their parents are former Khmer Rouge,” Phen Soeurm said. In schools throughout the Kingdom, the introduction of KR-related material has been a sensitive project. Prior to last year, high school history tests drew from a textbook that gave short shrift to the regime and its history, omitting some of the most basic facts about it. But on the 2010 national history exam, five of the 14 questions dealt with the Khmer Rouge period. In addition to identifying regime leaders, students are asked to explain why it is said that Tuol Sleng prison was a tragedy for the Cambodian people; who was behind Tuol Sleng; how the administrative zones of Democratic Kampuchea were organised; and when the regime was in power. These new additions to the exam follow the 2007 introduction of a government-approved textbook created by the Documentation Centre of Cambodia titled A History of Democratic Kampuchea. -

Cambodia-10-Contents.Pdf
©Lonely Planet Publications Pty Ltd Cambodia Temples of Angkor p129 ^# ^# Siem Reap p93 Northwestern Eastern Cambodia Cambodia p270 p228 #_ Phnom Penh p36 South Coast p172 THIS EDITION WRITTEN AND RESEARCHED BY Nick Ray, Jessica Lee PLAN YOUR TRIP ON THE ROAD Welcome to Cambodia . 4 PHNOM PENH . 36 TEMPLES OF Cambodia Map . 6 Sights . 40 ANGKOR . 129 Cambodia’s Top 10 . 8 Activities . 50 Angkor Wat . 144 Need to Know . 14 Courses . 55 Angkor Thom . 148 Bayon 149 If You Like… . 16 Tours . 55 .. Sleeping . 56 Baphuon 154 Month by Month . 18 . Eating . 62 Royal Enclosure & Itineraries . 20 Drinking & Nightlife . 73 Phimeanakas . 154 Off the Beaten Track . 26 Entertainment . 76 Preah Palilay . 154 Outdoor Adventures . 28 Shopping . 78 Tep Pranam . 155 Preah Pithu 155 Regions at a Glance . 33 Around Phnom Penh . 88 . Koh Dach 88 Terrace of the . Leper King 155 Udong 88 . Terrace of Elephants 155 Tonlé Bati 90 . .. Kleangs & Prasat Phnom Tamao Wildlife Suor Prat 155 Rescue Centre . 90 . Around Angkor Thom . 156 Phnom Chisor 91 . Baksei Chamkrong 156 . CHRISTOPHER GROENHOUT / GETTY IMAGES © IMAGES GETTY / GROENHOUT CHRISTOPHER Kirirom National Park . 91 Phnom Bakheng. 156 SIEM REAP . 93 Chau Say Tevoda . 157 Thommanon 157 Sights . 95 . Spean Thmor 157 Activities . 99 .. Ta Keo 158 Courses . 101 . Ta Nei 158 Tours . 102 . Ta Prohm 158 Sleeping . 103 . Banteay Kdei Eating . 107 & Sra Srang . 159 Drinking & Nightlife . 115 Prasat Kravan . 159 PSAR THMEI P79, Entertainment . 117. Preah Khan 160 PHNOM PENH . Shopping . 118 Preah Neak Poan . 161 Around Siem Reap . 124 Ta Som 162 . TIM HUGHES / GETTY IMAGES © IMAGES GETTY / HUGHES TIM Banteay Srei District . -

Consultative Workshop on Peam Krasop Wildlife Sanctuary Management Planning
Consultative Workshop on Peam Krasop Wildlife Sanctuary Management Planning Koh Kong City Hotel, Koh Kong Province, 21-22 November 2012 Organized by the Ministry of Environment, Koh Kong provincial Hall and IUCN INTERNATIONAL UNION FOR CONSERVATION OF NATURE Funded by Partners Consultative Workshop on Peam Krasop Wildlife Sanctuary Management Planning Koh Kong City Hotel, Koh Kong Province, 21-22 November 2012 Organized by the Ministry of Environment, Koh Kong provincial Hall and IUCN TABLE OF CONTENTS I. INTRODUCTION ................................................................................................................ 2! II. OBJECTIVES OF THE WORKSHOP ................................................................................ 2! III. PARTICIPANTS ............................................................................................................... 2! IV. OUTCOME OF THE WORKSHOP .................................................................................. 3! 4.1. Welcome Remarks by Mr Man Phala, Acting Director of the Koh Kong Provincial Environmental Department .............................................................................................. 3! 4.2. Welcome Remarks by Robert Mather, Head of Southeast Asia Group, IUCN ............... 3! 4.3. Welcome Remarks by H.E. Say Socheat, Deputy Governor of Koh Kong Province ...... 4! 4.4. Opening Speech by Mr Kim Nong, Deputy Director of the General Department of Administration for Nature Conservation and Protection, Ministry of Environment ......... 5! -

Biodiversity, Evolution and Ecological Specialization of Baculoviruses: A
Biodiversity, Evolution and Ecological Specialization of Baculoviruses: A Treasure Trove for Future Applied Research Julien Thézé, Carlos Lopez-Vaamonde, Jenny Cory, Elisabeth Herniou To cite this version: Julien Thézé, Carlos Lopez-Vaamonde, Jenny Cory, Elisabeth Herniou. Biodiversity, Evolution and Ecological Specialization of Baculoviruses: A Treasure Trove for Future Applied Research. Viruses, MDPI, 2018, 10 (7), pp.366. 10.3390/v10070366. hal-02140538 HAL Id: hal-02140538 https://hal.archives-ouvertes.fr/hal-02140538 Submitted on 26 May 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution| 4.0 International License viruses Article Biodiversity, Evolution and Ecological Specialization of Baculoviruses: A Treasure Trove for Future Applied Research Julien Thézé 1,2, Carlos Lopez-Vaamonde 1,3 ID , Jenny S. Cory 4 and Elisabeth A. Herniou 1,* ID 1 Institut de Recherche sur la Biologie de l’Insecte, UMR 7261, CNRS—Université de Tours, 37200 Tours, France; [email protected] (J.T.); [email protected] -

Intro Outline
THE REPRODUCTIVE ECOLOGY OF TWO TERRESTRIAL ORCHIDS, CALADENIA RIGIDA AND CALADENIA TENTACULATA RENATE FAAST Submitted for the degree of Doctor of Philosophy School of Earth and Environmental Sciences The University of Adelaide, South Australia December, 2009 i . DEcLARATION This work contains no material which has been accepted for the award of any other degree or diploma in any university or other tertiary institution to Renate Faast and, to the best of my knowledge and belief, contains no material previously published or written by another person, except where due reference has been made in the text. I give consent to this copy of my thesis when deposited in the University Library, being made available for loan and photocopying, subject to the provisions of the Copyright Act 1968. The author acknowledges that copyright of published works contained within this thesis (as listed below) resides with the copyright holder(s) of those works. I also give permission for the digital version of my thesis to be made available on the web, via the University's digital research repository, the Library catalogue, the Australasian Digital Theses Program (ADTP) and also through web search engines. Published works contained within this thesis: Faast R, Farrington L, Facelli JM, Austin AD (2009) Bees and white spiders: unravelling the pollination' syndrome of C aladenia ri gída (Orchidaceae). Australian Joumal of Botany 57:315-325. Faast R, Facelli JM (2009) Grazrngorchids: impact of florivory on two species of Calademz (Orchidaceae). Australian Journal of Botany 57:361-372. Farrington L, Macgillivray P, Faast R, Austin AD (2009) Evaluating molecular tools for Calad,enia (Orchidaceae) species identification. -

Comparative Methods Offer Powerful Insights Into Social Evolution in Bees Sarah Kocher, Robert Paxton
Comparative methods offer powerful insights into social evolution in bees Sarah Kocher, Robert Paxton To cite this version: Sarah Kocher, Robert Paxton. Comparative methods offer powerful insights into social evolution in bees. Apidologie, Springer Verlag, 2014, 45 (3), pp.289-305. 10.1007/s13592-014-0268-3. hal- 01234748 HAL Id: hal-01234748 https://hal.archives-ouvertes.fr/hal-01234748 Submitted on 27 Nov 2015 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Apidologie (2014) 45:289–305 Review article * INRA, DIB and Springer-Verlag France, 2014 DOI: 10.1007/s13592-014-0268-3 Comparative methods offer powerful insights into social evolution in bees 1 2 Sarah D. KOCHER , Robert J. PAXTON 1Department of Organismic and Evolutionary Biology, Museum of Comparative Zoology, Harvard University, Cambridge, MA, USA 2Institute for Biology, Martin-Luther-University Halle-Wittenberg, Halle, Germany Received 9 September 2013 – Revised 8 December 2013 – Accepted 2 January 2014 Abstract – Bees are excellent models for studying the evolution of sociality. While most species are solitary, many form social groups. The most complex form of social behavior, eusociality, has arisen independently four times within the bees. -

Somerset's Ecological Network
Somerset’s Ecological Network Mapping the components of the ecological network in Somerset 2015 Report This report was produced by Michele Bowe, Eleanor Higginson, Jake Chant and Michelle Osbourn of Somerset Wildlife Trust, and Larry Burrows of Somerset County Council, with the support of Dr Kevin Watts of Forest Research. The BEETLE least-cost network model used to produce Somerset’s Ecological Network was developed by Forest Research (Watts et al, 2010). GIS data and mapping was produced with the support of Somerset Environmental Records Centre and First Ecology Somerset Wildlife Trust 34 Wellington Road Taunton TA1 5AW 01823 652 400 Email: [email protected] somersetwildlife.org Front Cover: Broadleaved woodland ecological network in East Mendip Contents 1. Introduction .................................................................................................................... 1 2. Policy and Legislative Background to Ecological Networks ............................................ 3 Introduction ............................................................................................................... 3 Government White Paper on the Natural Environment .............................................. 3 National Planning Policy Framework ......................................................................... 3 The Habitats and Birds Directives ............................................................................. 4 The Conservation of Habitats and Species Regulations 2010 ..................................