The European IP Bulletin
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Official Journal C 244 of the European Union
Official Journal C 244 of the European Union Volume 59 English edition Information and Notices 5 July 2016 Contents II Information INFORMATION FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES European Commission 2016/C 244/01 Communication from the Commission amending the Annex to the Communication to the Member States on the application of Articles 107 and 108 of the Treaty on the Functioning of the European Union to short-term export-credit insurance ............................................................................. 1 IV Notices NOTICES FROM EUROPEAN UNION INSTITUTIONS, BODIES, OFFICES AND AGENCIES European Commission 2016/C 244/02 Euro exchange rates .............................................................................................................. 3 2016/C 244/03 Commission notice on the customs enforcement of Intellectual Property Rights concerning goods brought into the customs territory of the Union without being released for free circulation including goods in transit .................................................................................................................... 4 2016/C 244/04 Commission notice concerning the date of application of the Regional Convention on pan-Euro- Mediterranean preferential rules of origin or the protocols on rules of origin providing for diagonal cumulation between the Contracting Parties to this Convention .................................................... 10 EN NOTICES FROM MEMBER STATES 2016/C 244/05 United Kingdom Government notice concerning -

City Research Online
City Research Online City, University of London Institutional Repository Citation: McDonagh, L. and Mimler, M. (2017). Intellectual Property Law and Brexit: A Retreat or a Reaffirmation of Jurisdiction? In: Dougan, M. (Ed.), The UK after Brexit. (pp. 159-179). Cambridge, UK: Intersentia. ISBN 1780684711 This is the accepted version of the paper. This version of the publication may differ from the final published version. Permanent repository link: https://openaccess.city.ac.uk/id/eprint/17634/ Link to published version: Copyright: City Research Online aims to make research outputs of City, University of London available to a wider audience. Copyright and Moral Rights remain with the author(s) and/or copyright holders. URLs from City Research Online may be freely distributed and linked to. Reuse: Copies of full items can be used for personal research or study, educational, or not-for-profit purposes without prior permission or charge. Provided that the authors, title and full bibliographic details are credited, a hyperlink and/or URL is given for the original metadata page and the content is not changed in any way. City Research Online: http://openaccess.city.ac.uk/ [email protected] CHAPTER 8 INTELLECTUAL PROPERTY LAW AND BREXIT: A RETREAT OR A REAFFIRMATION OF JURISDICTION? Luke McDonagh and Marc Mimler* Intellectual Property Law and Brexit 1. INTRODUCTION The effect of European Union law on intellectual property (IP) law in the United Kingdom has been profound. There is no area of IP law that does not feature EU legislation or CJEU case law. In fact, it may be the most ‘Europeanised’ area of private law.1 For this reason, ‘Brexit’ will undoubtedly have a massive impact on the current IP framework in the UK. -

Eucrim 1/2016
eucrim 2016 /1 THE EUROPEAN CRIMINAL LAW ASSOCIATIONS‘ FORUM Focus: Procedural Rights and Cooperation – New Tendencies Dossier particulier: Droits procéduraux et coopération – nouvelles tendances Schwerpunktthema: Verfahrensgarantien und Zusammenarbeit – neue Tendenzen The Directive on the Presumption of Innocence and the Right to Be Present at Trial Steven Cras and Anže Erbežnik The Directive on the Presumption of Innocence. A Missed Opportunity for Legal Persons? Stijn Lamberigts Inaudito reo Proceedings, Defence Rights, and Harmonisation Goals in the EU Prof. Dr. Stefano Ruggeri Paving the Way for Improved Mutual Assistance in the Context of Customs Fraud Emilia Porebska Können die Regelungen über die Zusammenarbeit der EU-Mitgliedstaaten bei der Strafverfolgung kurzerhand aufgehoben werden? Ulrich Schulz Vollstreckungshilfe zwischen Deutschland und Taiwan auf neuer Grundlage Dr. Ralf Riegel and Dr. Franca Fülle 2016 / 1 ISSUE / ÉDITION / AUSGABE The Associations for European Criminal Law and the Protection of Financial Interests of the EU is a network of academics and practitioners. The aim of this cooperation is to develop a European criminal law which both respects civil liberties and at the same time protects European citizens and the European institutions effectively. Joint seminars, joint research projects and annual meetings of the associations’ presidents are organised to achieve this aim. Contents News* Articles European Union Procedural Rights and Cooperation – New Tendencies Foundations Procedural Criminal Law 25 The Directive on the Presumption of 2 Fundamental Rights 13 Procedural Safeguards Innocence and the Right to Be Present at 2 Area of Freedom, Security 13 Data Protection Trial. Genesis and Description of the New and Justice 15 Ne bis in idem EU-Measure 3 Schengen Steven Cras and Anže Erbežnik Cooperation 36 The Directive on the Presumption of In- Institutions 16 European Arrest Warrant nocence. -

Information Disclosure Mechanism for Technological Protection Measures in China
Journal of Intellectual Property Rights Vol 17, November 2012, pp 532-538 Information Disclosure Mechanism for Technological Protection Measures in China Lili Zhao† Center for China Information Security Law, Xi’an Jiao tong University, Shaanxi Xi’an, P R China 710 049 Received 12 February 2012, revised 21 May 2012 With increasing cases of digital works being copied and pirated, technological protection measures have been greatly favoured by copyright owners for protecting the intellectual property in their digital works, while ensuring that these works can be used and disseminated. However, when any copyright owner or supplier fails to disclose the information of technological protection measures appropriately or effectively, damages such as privacy violations, security breaches and unfair competition may be caused to the public. Therefore, it is necessary to establish an information disclosure mechanism for technological protection measures, make the labeling obligation with regard to technological protection measures by copyright owners apparent and warning to security risks obligatory by legislation; effectively guarding against information security threats from the technological protection measures. Keywords: Technological protection measures, TPM, information disclosure, label and warning The advance of digital technologies make many information security, it is necessary to integrate such acts things become possible, including the copy and in a standardized, normalized and legal manner. dissemination of commercially valuable digital works Therefore, to further prevent copyright owners from through global digital network. Particularly, among using insecure or untested software, protect information the copyright owners of entertainment industry, security and prohibit the abuse of TPMs; disclosure technological protection measures (TPMs) have been obligations should be established for the right holders of regarded as a necessary creation to help digital works TPMs in the form of legislation. -
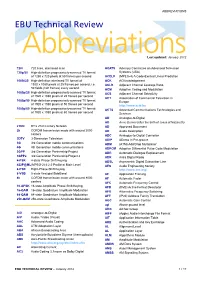
ABBREVIATIONS EBU Technical Review
ABBREVIATIONS EBU Technical Review AbbreviationsLast updated: January 2012 720i 720 lines, interlaced scan ACATS Advisory Committee on Advanced Television 720p/50 High-definition progressively-scanned TV format Systems (USA) of 1280 x 720 pixels at 50 frames per second ACELP (MPEG-4) A Code-Excited Linear Prediction 1080i/25 High-definition interlaced TV format of ACK ACKnowledgement 1920 x 1080 pixels at 25 frames per second, i.e. ACLR Adjacent Channel Leakage Ratio 50 fields (half frames) every second ACM Adaptive Coding and Modulation 1080p/25 High-definition progressively-scanned TV format ACS Adjacent Channel Selectivity of 1920 x 1080 pixels at 25 frames per second ACT Association of Commercial Television in 1080p/50 High-definition progressively-scanned TV format Europe of 1920 x 1080 pixels at 50 frames per second http://www.acte.be 1080p/60 High-definition progressively-scanned TV format ACTS Advanced Communications Technologies and of 1920 x 1080 pixels at 60 frames per second Services AD Analogue-to-Digital AD Anno Domini (after the birth of Jesus of Nazareth) 21CN BT’s 21st Century Network AD Approved Document 2k COFDM transmission mode with around 2000 AD Audio Description carriers ADC Analogue-to-Digital Converter 3DTV 3-Dimension Television ADIP ADress In Pre-groove 3G 3rd Generation mobile communications ADM (ATM) Add/Drop Multiplexer 4G 4th Generation mobile communications ADPCM Adaptive Differential Pulse Code Modulation 3GPP 3rd Generation Partnership Project ADR Automatic Dialogue Replacement 3GPP2 3rd Generation Partnership -

The German Association for the Protection of Intellectual Property (GRUR)
The German Association for the Protection of Intellectual Property (GRUR) The Secretary General German Association for the Protection of Intellectual Property (GRUR) Konrad-Adenauer-Ufer 11 Konrad-Adenauer-Ufer 11 . RheinAtrium . 50668 Köln . Germany RheinAtrium 50668 Köln Phone +49 (0) 221 650 65-151 Fax +49 (0) 221 650 65-205 Email [email protected] www.grur.org July 1, 2013 Full English Version Opinion of the German Association for the Protection of Intellectual Property (Deutsche Vereinigung für gewerblichen Rechtsschutz und Urheberrecht e.V.) regarding the European Commission proposal for a recast of the Trade Mark Directive The German Association for the Protection of Intellectual Property (GRUR) is a scientific non-profit as- sociation. Its statutory purpose is the academic advancement and development of industrial property, copyright and competition law at the German, European and international level. For fulfilling these tasks, GRUR provides assistance to the legislative bodies and to authorities competent for issues of intellectu- al property law, organises conferences, workshops and further education courses, provides financial aid to selected university chairs and research projects and also publishes four leading German professional IP law journals (GRUR, GRUR International, GRUR-RR and GRUR-Prax.) With over 5,250 members coming from 52 countries, the association offers an umbrella for a wide range of IP professionals: law- yers, patent attorneys, judges, academics, representatives of the specific public authorities and of the international organisations as well as enterprises dealing with issues of intellectual property. On 27 March 2013 the European Commission presented proposals for an amendment of Regulation 207/2009 on the Community trade mark (Regulation) and for a recast of Directive 2008/95/EC for the approximation of the laws of the Member States relating to trade marks (Directive). -

Intellectual Property Part 2 Pornography
Intellectual Property Part 2 Pornography By Jeremy Parmenter What is pornography Pornography is the explicit portrayal of sexual subject matter Can be found as books, magazines, videos The web has images, tube sites, and pay sites scattered with porn Statistics 12% of total websites are pornography websites 25% of total daily search engine requests are pornographic requests 42.7% of internet users who view pornography 34% internet users receive unwanted exposure to sexual material $4.9 billion in internet pornography sales 11 years old is the average age of first internet exposure to pornography Innovations ● Richard Gordon created an e-commerce start up in mid- 90s that was used on many sites, most notably selling Pamela Anderson/Tommey Lee sex tape ● Danni Ashe founded Danni's Hard Drive with one of the first streaming video without requiring a plug-in ● Adult content sites were one of the first to use traffic optimization by linking to similar sites ● Live chat during the early days of the web ● Pornographic companies were known to give away broadband devices to promote faster connections Negative Impacts ● Between 2001-2002 adult-oriented spam rose 450% ● Malware such as Trojans and video codecs occur most often on porn sites ● Domain hijacking, using fake documents and information to steal a site ● Pop-ups preventing users from leaving the site or infecting their computer ● Browser hijacking adware or spyware manipulating the browser to change home page or search engine to a bogus site, including pay-per-click adult site ● Accessibility -

Governance by Committee: the Role of Committees in European Policy Making and Policy
Governance by Committee: The Role of Committees in European Policy Making and Policy Research Paper 00/GHA Return to Introduction STATE OF THE ART REPORT CONTRACT NUMBER: HPSE-CT-1999-00019 PROJECT NUMBER: SERD-1999-00128 TITLE: GOVERNANCE BY COMMITTEE, THE ROLE OF COMMITTEES IN EUROPEAN POLICY-MAKING AND POLICY IMPLEMENTATION MAASTRICHT, MAY 2000 Table of Contents 1. General Introduction ................................................................................................................ 4 Subproject 1: The Standing Committees in the European Parliament 2.1..................Introduction ............................................................................................................... 6 2.2..................The Evolution of the European Parliament: From Consultative ............................... 6 Assembly to Co-legislator 2.2.1...............The EP as a Legislative Actor after Maastricht......................................................... 7 2.2.2...............EP and Council on an Even Footing after Amsterdam.............................................. 8 2.2.2.1. ..........The Streamlining of the Co-decision Procedure........................................................ 9 2.3..................The Role of EP Committees in the Legislative Process ............................................ 10 2.3.1...............Membership in EP Committees.................................................................................12 2.3.2...............Powers and Competences of EP Committees........................................................... -

The Repackaging of Pharmaceutical Products and Parallel Trade in the EU
Legal Feature The Repackaging of Pharmaceutical Products and Parallel Trade in the EU Héctor Armengod and Laura Melusine Baudenbacher examine how European case law affects trademark owners’ ability to lawfully oppose further marketing of their repackaged product. The question of whether pharmaceutical products can be repackaged by parallel traders has been – and continues to be – a recurrent topic before the European Court of Justice. In the European Union, each member state, based on its own healthcare policy, dictates Price differences among the prices of the drugs sold in its territory. This leads to significant differences in the price of EU member states lead to pharmaceuticals across the EU. These differences create business opportunities for parallel business opportunities for importers who can buy the drugs in those countries where they are cheaper and import them in parallel importers the more expensive ones, thereby obtaining a lucrative margin. The packaging and labelling of pharmaceuticals is highly regulated both at EU and member state level, and a product acquired in a given member state often needs to be repackaged before it is placed on the market of a different member state. Repackaging can interfere with the trademark rights of the original supplier of the product. The protection of trademark rights has been recognised by the ECJ as an essential element in the system of undistorted competition which the EC Treaty seeks to establish and maintain1. The exercise of these rights can, however, be in conflict with the principle of free movement of goods, which is one of the cornerstones of the EU internal market. -

Collective Rights Management in the Digital Single Market
Implementation of the EU Trade Mark Directive 2015 Intellectual Property Office is an operating name of the Patent Office Implementation of the EU Trade Mark Directive 2015 Contents Introduction ............................................................................................................. 1 Relevance of UK withdrawal from the EU ......................................................... 1 Our approach to implementation ...................................................................... 2 About this consultation ............................................................................................ 2 Proposed amendments in detail ............................................................................. 3 Article 3: Signs of which a trade mark may consist .......................................... 3 Article 3: Removal of requirement for ‘graphic representation’ in section 1 - new file formats ................................................................. 3 Article 3 (and Article 39.2): ‘Competent authorities’ ............................... 4 Article 4: Absolute grounds for refusal ............................................................ 5 Article 4.1(i),(j),(k) & (l) – specific references to geographical indications, traditional terms for wine, etc .............................................. 5 Article 4.5: distinctive character acquired after application date, but before registration date (optional) ............................................ 6 Article 5: Relative Grounds for refusal or invalidity .......................................... -

EU Trade Marks: When Is the Right Time for a Face Lift?
The In-House Lawyer www.inhouselawyer.co.uk The In-House Lawyer Spring 2016 TMT Focus EU trade marks: when is the right time for a face lift? By John R Olsen, partner, Locke Lord t appears that the right time for a face lift is conceived EU trade mark and the Office for when the spirit needs a nuance of rejuvenation Harmonization in the Internal Market (OHIM) and the elements for doing so are all to hand. itself, which is to be renamed the European Union ISimilarly, as a brand is the face of an organisation Intellectual Property Office (EUIPO) . The new the time for a ‘face lift’ of a business organisation is Directive came into force on 13 January 2016, when opportunities present themselves and there is and is intended to harmonise further the national a need to revitalise. The European Parliament has trade mark systems and laws of the individual now provided that opportunity and necessity for member states of the European Union. businesses to think seriously about getting a new face for their brands in view of the new legislation. Summary of key administrative changes and alterations in examination practice John R Olsen Introduction Pricing At the end of December 2015, the European Of importance to smaller companies, individuals Partner, Locke Lord Parliament adopted a reform package for European and start-ups is the abolition of the previously [email protected] trade marks which will result ultimately in the inflexible ‘three classes for one’ pricing structure, implementation of substantial changes to European whereby the cost of filing for a CTM in up to trade mark law throughout the course of 2016 – and three classes was fixed, so that an application beyond. -

Of Price Discriminiation, Rootkits and Flatrates
Of Price Discriminiation, Rootkits and Flatrates Volker Grassmuck Helmholtz-Zentrum für Kulturtechnik, Humboldt-University Berlin 19 February 2006 DRAFT VERSION – final version is slated for print publication Licensed under Creative Commons Share-Alike Germany 2.0 „Copyright owners continue to be ambivalent about the Internet. On the one hand, it represents a fantastic new medium for distribution; on the other, many in the publishing industry see it as one ‚giant, out of control copying machine.‘ ... The very technological advances that make rights management more difficult – the dramatic reduction in costs of copying and distribution – also offer a fantastic opportunity for owners of intellectual content.“1 Without scarcity there is no market. Information by its nature is a public good.2 Copyright law artificially creates scarcity by granting exclusive rights to it for a limited time. Media technology so far helped enforcability of those rights because the means of production and the means of distribution of informational goods were expensive and therefore scarce. The digital revolution does away with this scarcity. PC and Internet bring to virtually everyone the power of the printing press and the recording studio. Only now, information‘s defining qualities of non-rivalrousness and non-excludability come to full bearing. Zero cost for reproduction and distribution is indeed a fantastic value proposition for information vendors. Alas, it is undermined by the fact that for consumers the cost of copying and distribution is zero as well. Peer-to-peer networks show that transporting bits from A to B is now such a low-cost service that users can effortlessly provide it to each other.